Compound and preparation method thereof
A technology of compounds and base catalysts, applied in the field of active compounds and their preparation, can solve problems such as inability to meet the needs of medicines, and achieve the effects of reducing toxicity and achieving good effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
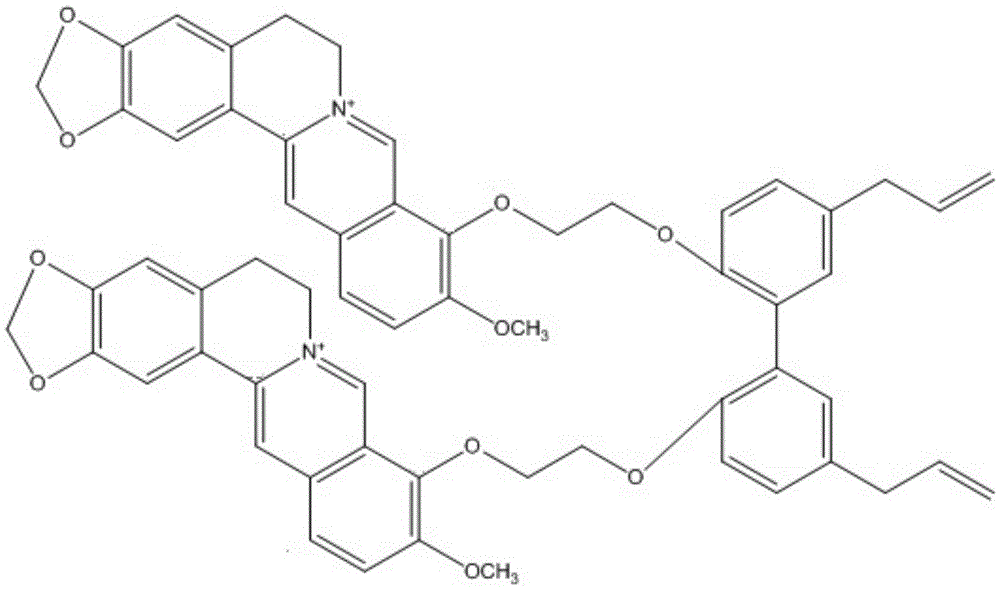
[0048] An embodiment of the compound of the present invention, the chemical structural formula of the compound described in this embodiment is as attached figure 1 As shown, the compound described in this example is prepared by the following preparation method:
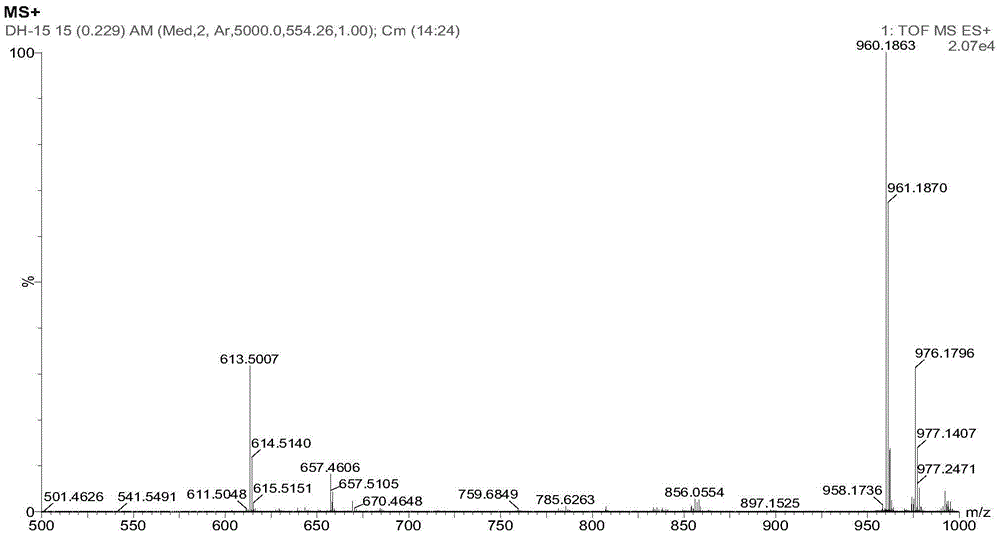
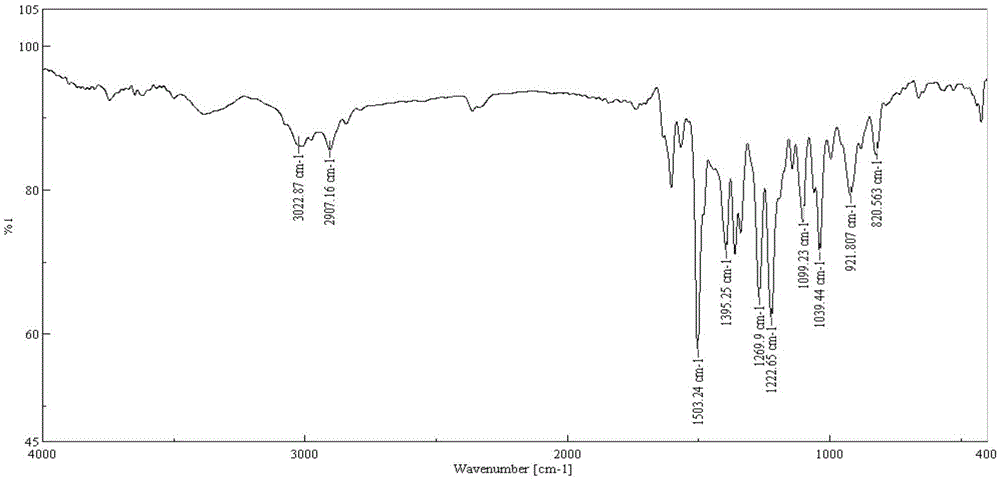
[0049] (1) Place the compound of formula (6) in a dry and clean three-necked flask, add HEPT, the molar ratio of HEPT to the compound of formula (5) is 5:1, heat and reflux reaction in the electric jacket, at a temperature of 50- The reaction was carried out at 400°C, monitored by HPLC during the reaction, and the reaction was stopped until the basic reaction of the raw materials was complete or the product no longer increased. When the temperature drops below 120°C, add pure ice water while stirring, place it in the refrigerator for crystallization (at a temperature of about 0°C), suction filter, wash, and dry after crystallization to obtain the formula (1) Compound; In this step, the yield of the compound of the ob...
Embodiment 2
[0054] An embodiment of the compound of the present invention, the chemical structural formula of the compound described in this embodiment is as attached figure 1 As shown, the compound described in this example is prepared by the following preparation method:
[0055] (1) Place the compound of formula (6) in a dry and clean three-necked flask, add DMAC, the molar ratio of DMAC to the compound of formula (5) is 6:1, and heat the reflux reaction in the electric jacket, at a temperature of 50 ~ The reaction was carried out at 400°C, monitored by HPLC during the reaction, and the reaction was stopped until the basic reaction of the raw materials was complete or the product no longer increased. When the temperature drops below 120°C, add pure ice water while stirring, place it in the refrigerator for static crystallization (at a temperature of about 0°C), filter with suction after crystallization, wash, and dry to obtain the formula (1) Compound; In this step, the yield of the c...
Embodiment 3
[0059] An embodiment of the compound of the present invention, the chemical structural formula of the compound described in this embodiment is as attached figure 1 As shown, the compound described in this example is prepared by the following preparation method:
[0060] (1) Place the compound of formula (6) in a dry and clean three-necked flask, add DMSO, the molar ratio of DMSO to the compound of formula (5) is 8:1, heat and reflux reaction in the electric jacket, at a temperature of 50 ~ The reaction was carried out at 400°C, monitored by HPLC during the reaction, and the reaction was stopped until the basic reaction of the raw materials was complete or the product no longer increased. When the temperature drops below 120°C, add pure ice water while stirring, place it in the refrigerator for crystallization (at a temperature of about 0°C), suction filter, wash, and dry after crystallization to obtain the formula (1) Compound; In this step, the yield of the compound of the o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com