Method and system for recording clinical scientific research data
A technology of clinical scientific research and recording methods, applied in the field of clinical scientific research, can solve problems such as poor data reliability, difficulty in satisfying authenticity and integrity, achieve high consistency and reliability, avoid observation differences and result differences, and reduce difficulty Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
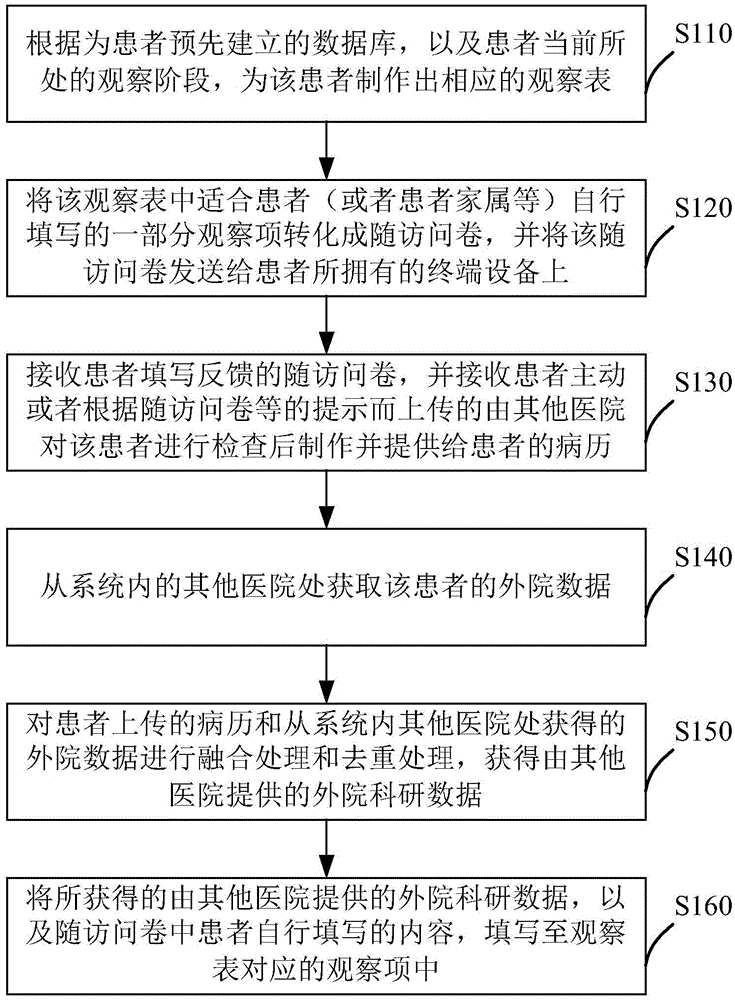
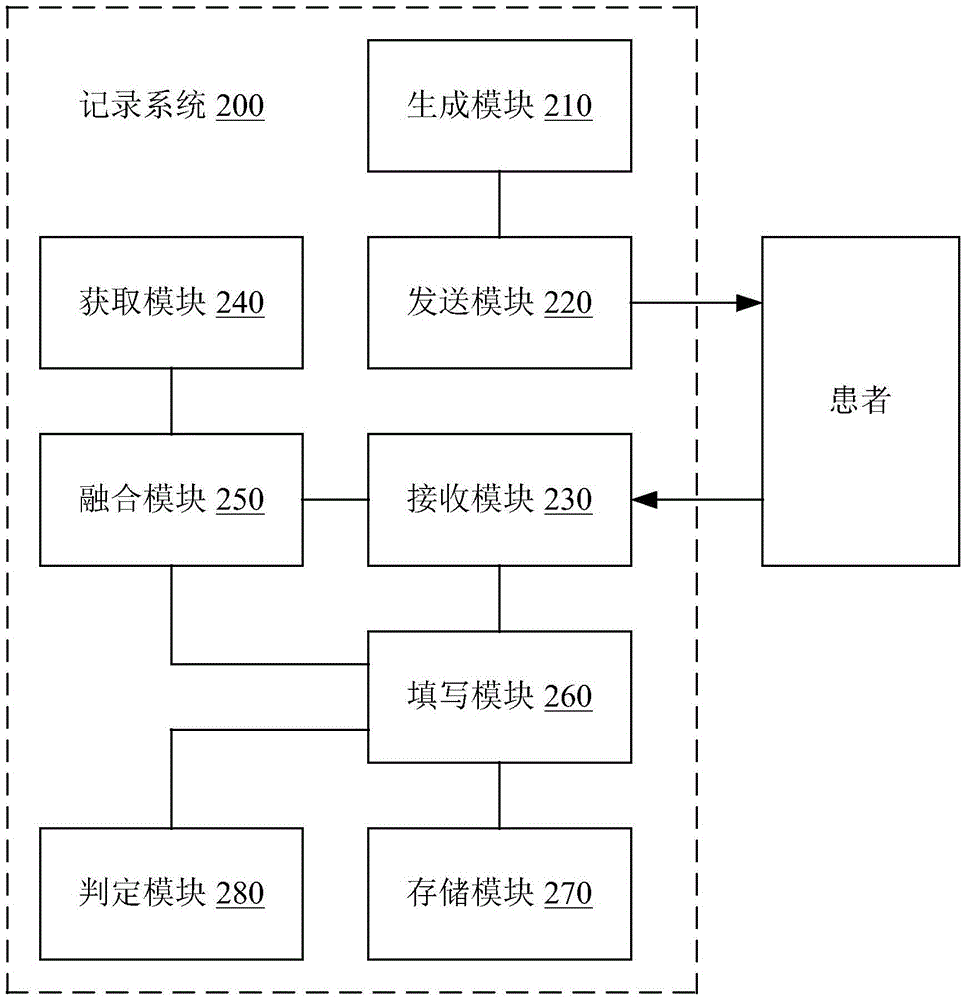
[0022] The implementation of the present invention will be described in detail below in conjunction with the accompanying drawings and examples, so as to fully understand and implement the implementation process of how to apply technical means to solve technical problems and achieve corresponding technical effects in the present invention. The embodiments of the present invention and the various features in the embodiments can be combined with each other under the premise of no conflict, and the formed technical solutions are all within the protection scope of the present invention.
[0023] In addition, the steps included in the methods of the embodiments of the present invention shown in the drawings can be executed in a computer system such as a set of computer-executable instructions. Moreover, although the method in the embodiment of the present invention shows a certain logical order of the technical solution of the present invention during execution in the flow chart sho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com