Novel neurokinin 1 receptor antagonist compounds ii
A compound and solvate technology, applied in the field of the new neurokinin 1 receptor antagonist compound II, can solve the problem of reducing systemic effects in the central nervous system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
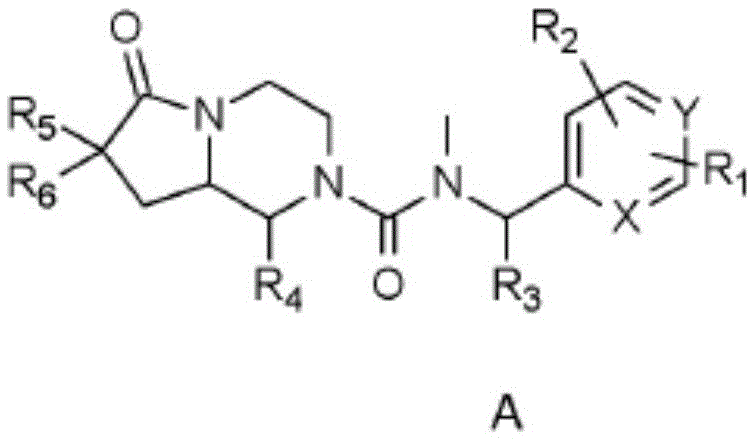
[0095] In one embodiment the present invention relates to a compound of general formula A(i) or a pharmaceutically acceptable salt or solvate thereof
[0096]
[0097] where R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , X and Y as shown above,
[0098] The condition is that when both X and Y are CH, R 4 Not 2-methylphenyl.
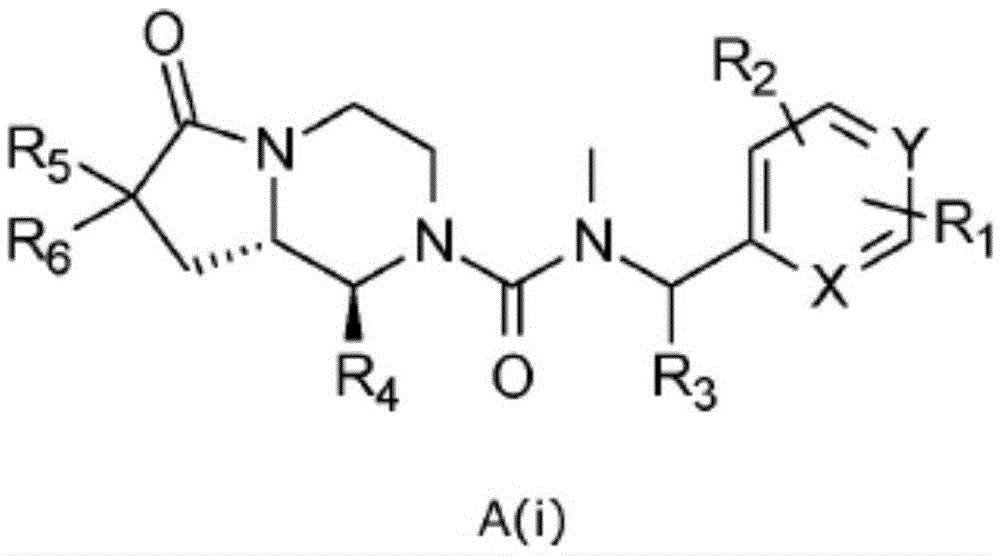
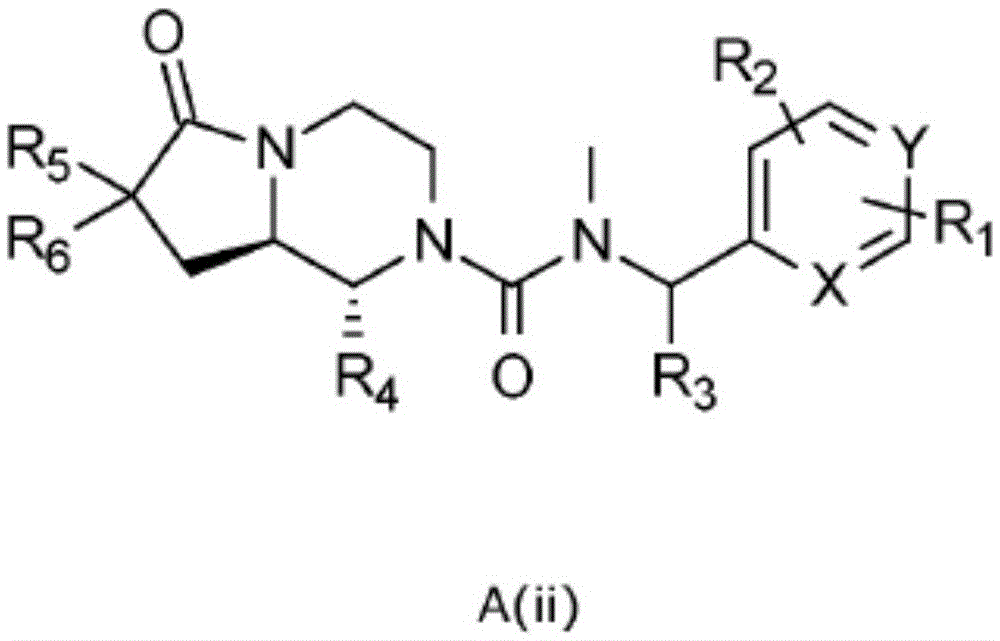
[0099] In one embodiment the present invention relates to a compound of general formula A(ii) or a pharmaceutically acceptable salt or solvate thereof
[0100]
[0101] where R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , X and Y as shown above,
[0102] The condition is that when both X and Y are CH, R 4 Not 2-methylphenyl.
[0103] In one embodiment the invention relates to compounds of general formula A, wherein R 1 and R 2 Indicates (C 1-4 ) Haloalkyl.
[0104] In one embodiment the invention relates to compounds of general formula A, wherein R 1 Indicates (C 1-4 ) haloalkyl and R 2 Indicates (C 1-4 )alkyl.
[0105] In one embodiment the inve...
Embodiment
[0420] Proton magnetic resonance (NMR) spectra were recorded on a Bruker instrument on a 400 MHz spectrometer at 25°C. Chemical shifts are reported in ppm (δ) using the residual solvent line as internal standard. Peak diversity is indicated as follows: s, singlet; d, doublet; dd, double doublet; t, triplet; dt, double triplet; q, quartet; quin, quintet; m, Multiplet; brs, broad singlet.
[0421] Total ion current (TIC) and DADUV chromatographic traces along with peak-related MS and UV spectra were also collected on a SHIMADZUL LCMS2020 system equipped with an LCMS2020 mass spectrometer operating in positive and / or negative electrospray ionization modes. [LC / MS / ESI(+ / -): analysis was performed using ChromolithSpeedRODC18 column (50x4.6mm, 2μm particle size), column temperature 35°C, mobile phase: A=CH3CN / H2O / FA=10 / 90 / 0.05 and B =CH3CN / H2O / FA=90 / 10 / 0.05, flow rate: 3.0mL / min, gradient: t=0.8min20%B, t=3.5min95%B, t=4.3min95%B, t=4.4min20%B.
[0422] Use the SHIMADZU6A system ...
Embodiment 3
[1119] Example 3: Compound 3
[1120] N-(3,5-bis(trifluoromethyl)benzyl)-1-(2,4-dimethylphenyl)-N-methyl-6-oxohexahydropyrrolo [1,2-a]pyrazine-2(1H)-carboxamide (mixture of trans isomers)
[1121]
[1122] To a solution of triphosgene (65 mg, 0.22 mmol) in EtOAc (10 mL) was added Intermediate 3 (mixture of trans, 120 mg, 0.49 mmol), DMAP (4 mg, 0.035 mmol) and TEA (209 μL, 1.5 mmol) at 0 °C ) in EtOAc (2 mL). The mixture was stirred at room temperature for 1.5 h before the addition of 1-(3,5-bis(trifluoromethyl)phenyl)-N-methylmethanamine (251 mg, 0.98 mmol) and TEA ( 107mg, 1.05mmol). The reaction was stirred at 45 °C for 48 h and washed with saturated NH 4 The Cl(aq) solution was quenched. The resulting mixture was extracted with EtOAc (2x20 mL). The combined organic layers were washed with brine, washed with anhydrous Na 2 SO 4 Dry and concentrate in high vacuum. The residue was purified by Prep-HPLC to give the title compound 3 (10 mg, 4%). 1 HNMR (600MHz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com