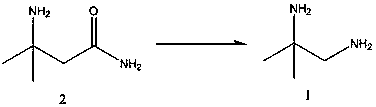
A new preparation method of anegliptin intermediate 1,2-diamino-2-methylpropane
A technology of methylpropane and diamino, which is applied in the field of anegliptin intermediate 1,2-diamino-2-methylpropane, which can solve the problems of high raw material prices, increased risk, difficulty and danger
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0008] In the reactor, add sodium hydroxide (153.7g, 3.84mol), water (1610ml) and cool down to 0-2°C, add bromine (192g, 1.2mol) at this temperature, and stir at this temperature for 30 minutes , add 3-amino-3-methylbutanamide (116.2g, 1mol), keep the same temperature and stir for 2 hours, then slowly raise the temperature to room temperature, stir for 1 hour, then raise the temperature to 70-72°C for 2 hours, cool down To room temperature, slowly add sodium hydroxide (340g) at room temperature, then extract with ethyl acetate (1000ml×4), combine the extracts, dehydrate with 30g of anhydrous sodium sulfate, filter, concentrate to remove ethyl acetate, and the remaining liquid Fractions at 113-115°C were collected to obtain 78.5 g of colorless 1,2-diamino-2-methylpropane with a purity of 98% and a yield of 89%.
Embodiment 2
[0010] In the reactor, add sodium hydroxide (120g, 3mol), water (1610ml) and cool down to 0-2°C, add bromine (153.6g, 0.96mol) at this temperature, and stir at this temperature for 30 minutes. Add 3-amino-3-methylbutanamide (116.2g, 1mol), keep the same temperature and stir for 2 hours, then slowly raise the temperature to room temperature, stir for 1 hour, then raise the temperature to 70-72°C for 2 hours, cool down to Keep at room temperature and slowly add sodium hydroxide (340g), then extract with ethyl acetate (1000ml×4), combine the extracts, dehydrate with 30g of anhydrous sodium sulfate, filter, concentrate to remove ethyl acetate, and collect the remaining liquid The fraction at 113-115°C yielded 67.9 g of colorless 1,2-diamino-2-methylpropane with a purity of 98% and a yield of 77%.
Embodiment 3
[0012] In the reactor, add sodium hydroxide (153.7g, 3.84mol), water (1610ml) and cool down to 5-10°C, add bromine (160g, 1.0mol) at this temperature, and stir at this temperature for 30 minutes , add 3-amino-3-methylbutanamide (116.2g, 1mol), keep the same temperature and stir for 2 hours, then slowly raise the temperature to room temperature, stir for 1 hour, then raise the temperature to 70-72°C for 2 hours, cool down To room temperature, slowly add sodium hydroxide (340g) at room temperature, then extract with ethyl acetate (1000ml×4), combine the extracts, dehydrate with 30g of anhydrous sodium sulfate, filter, concentrate to remove ethyl acetate, and the remaining liquid Fractions at 113-115°C were collected to obtain 48.5 g of colorless 1,2-diamino-2-methylpropane with a purity of 97.6% and a yield of 55%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com