Octapeptide modified dexamethasone, preparation, nano-structure and application thereof
A technology of dexamethasone and -his-gly-lys, which is applied in the field of biomedicine and can solve the problems of no obvious improvement in curative effect and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
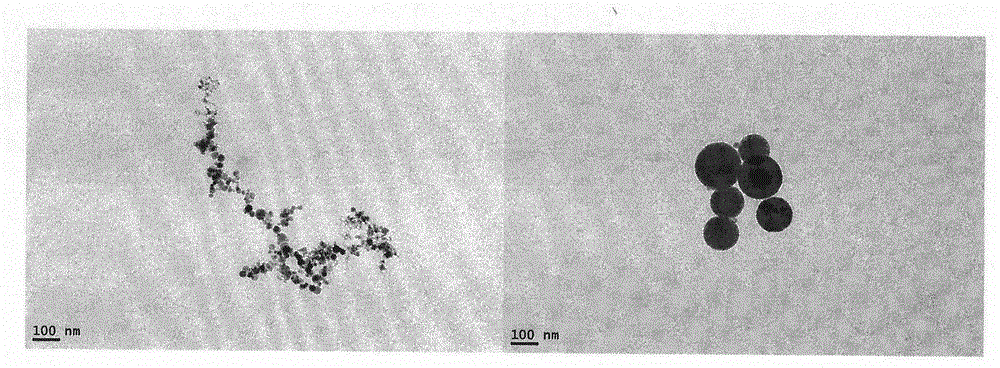
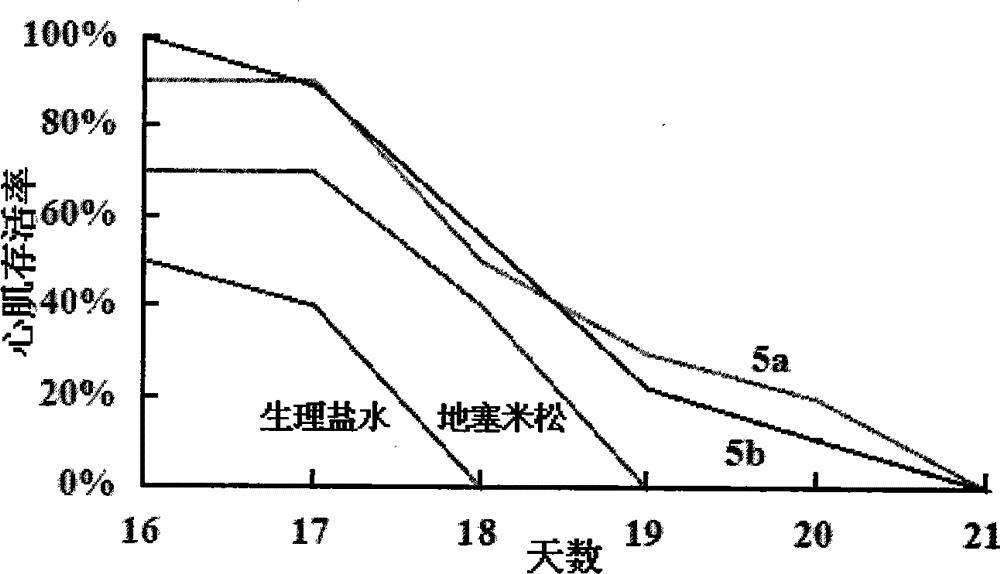
Image
Examples
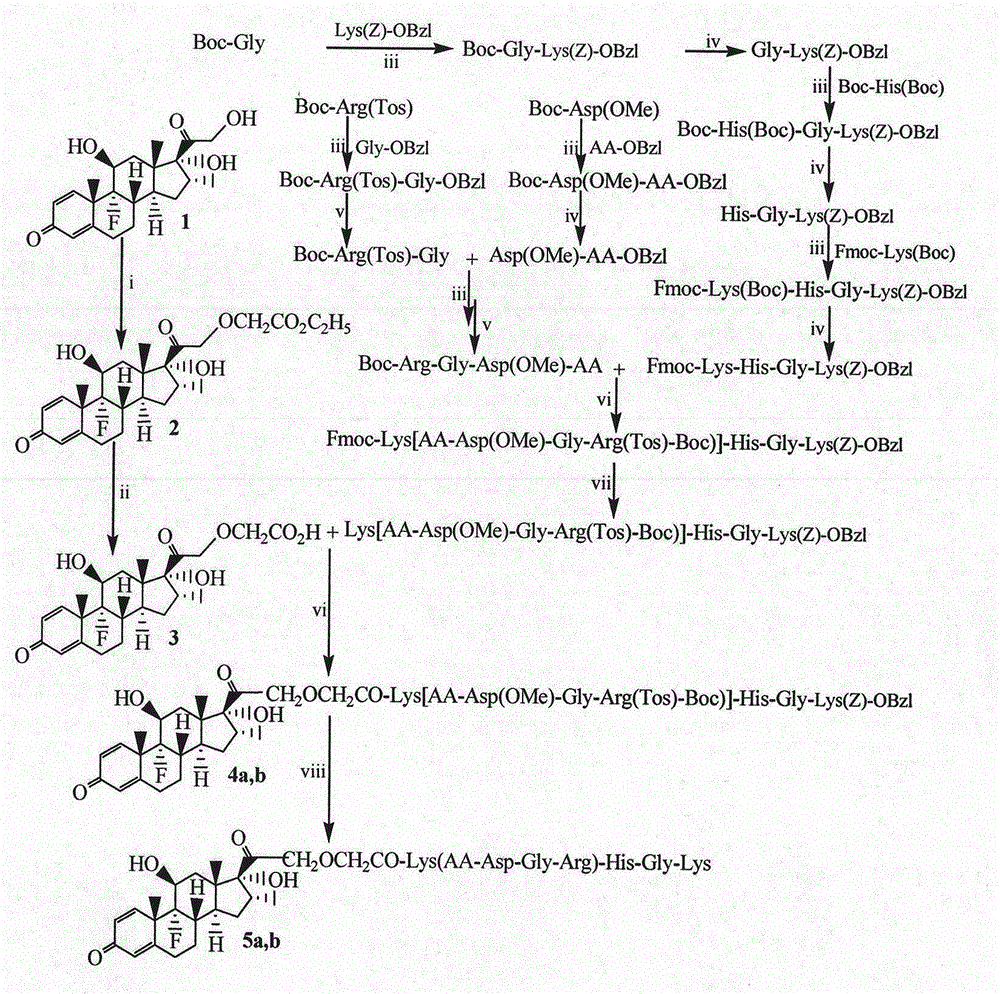
Embodiment 1
[0026] Example 1 Preparation of HCl Gly-Lys(Z)-OBzl
[0027] Weigh 1.154g (6.6mmol) Boc-Gly, 0.938g (6.9mmol) HOBt, dissolve in dry tetrahydrofuran (THF), stir under ice bath, add dropwise 1.614g (7.8mmol) DCC dissolved in THF, stir and activate for 30 Minutes later, add 3.000g (7.4mmol) HCl Lys(Z)-OBzl, adjust pH=8 with NMM, react at room temperature for 12 hours, TLC (CH 2 Cl 2 / MeOH=20 / 1), showing that the raw material point disappeared. DCU was removed by filtration, the filtrate was concentrated under reduced pressure, the residue was dissolved in 100 mL of ethyl acetate, and the obtained ethyl acetate solution was successively washed with 20 mL of saturated aqueous sodium bicarbonate, 20 mL of saturated aqueous sodium chloride, 20 mL of saturated aqueous potassium hydrogensulfate, and 20 mL of saturated aqueous chlorine Sodium chloride aqueous solution, 20mL saturated sodium bicarbonate aqueous solution, 20mL saturated sodium chloride aqueous solution were washed 3 tim...
Embodiment 2
[0028] Example 2 Preparation of Boc-His(Boc)-Gly-Lys(Z)-OBzl
[0029] Weigh 4.894g (13.8mmol) Boc-His (Boc), 1.960g (14.7mmol) HOBt, dissolve in dry tetrahydrofuran (THF), stir under ice bath, add dropwise 3.408g (16.5mmol) DCC dissolved in THF, After stirring and activating for 30 minutes, add 8.200 g (16.4 mmol) of HCl Gly-Lys(Z)-OBzl, adjust the pH to 8 with NMM, react at room temperature for 12 hours, TLC (CH 2 Cl 2 / MeOH=20 / 1), showing that the raw material point disappeared. DCU was removed by filtration, the filtrate was concentrated under reduced pressure, the residue was dissolved in 200 mL of ethyl acetate, and the obtained ethyl acetate solution was sequentially washed with 50 mL of saturated aqueous sodium bicarbonate, 50 mL of saturated aqueous sodium chloride, 50 mL of saturated aqueous potassium hydrogensulfate, and 50 mL of saturated aqueous chlorine Aqueous sodium chloride solution, 50 mL saturated aqueous sodium bicarbonate solution, and 50 mL saturated aqu...
Embodiment 3
[0030] Example 3 Preparation of HCl His-Gly-Lys(Z)-OBzl
[0031]To 10.0g (12.5mmol) Boc-His(Boc)-Gly-Lys(Z)-OBzl, add 100mL of 4M ethyl acetate solution of hydrogen chloride under ice bath, after reacting for 2 hours, TLC (CH 2 Cl 2 / MeOH=20 / 1) showed disappearance of starting point. Concentrate under reduced pressure. The residue was dissolved in anhydrous ethyl acetate and concentrated under reduced pressure. This operation was repeated 3 times. The residue was dissolved in anhydrous ether and concentrated under reduced pressure. This operation was also repeated 3 times. Yield 7.5 g (94.9%) of the title compound.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com