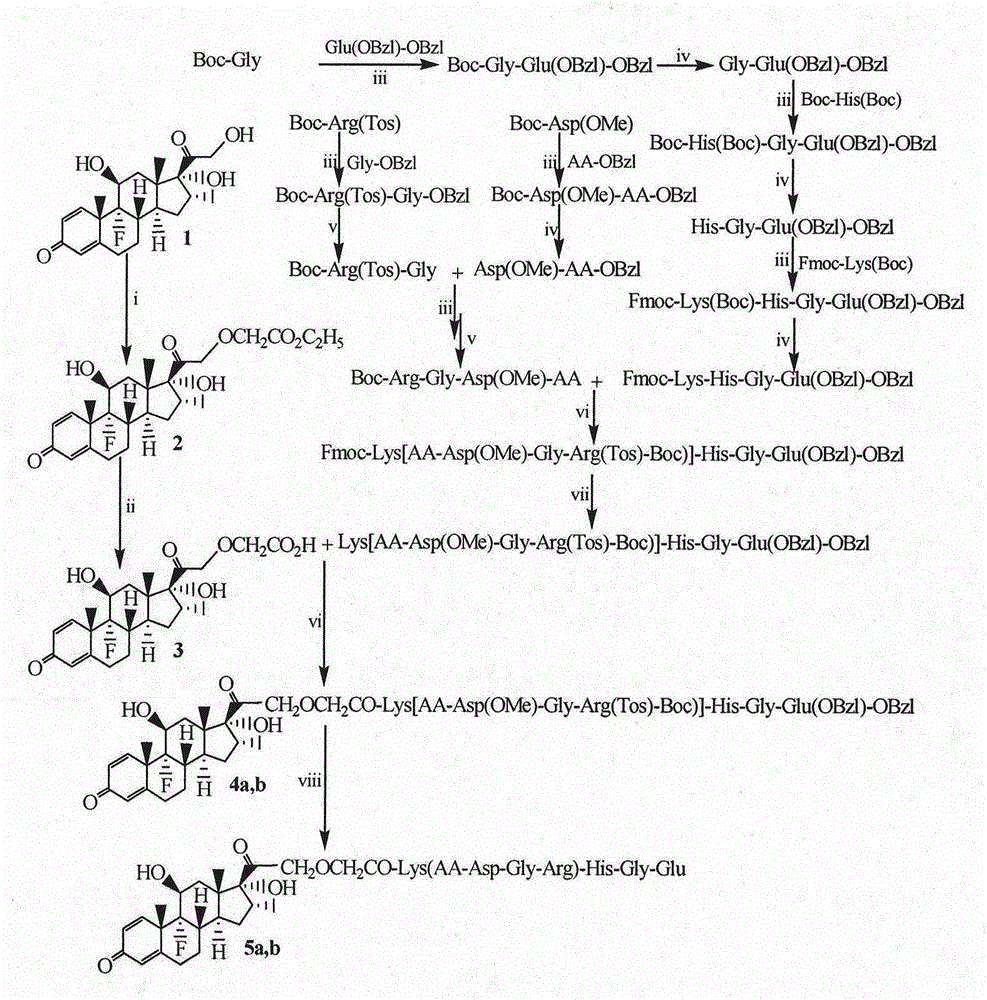
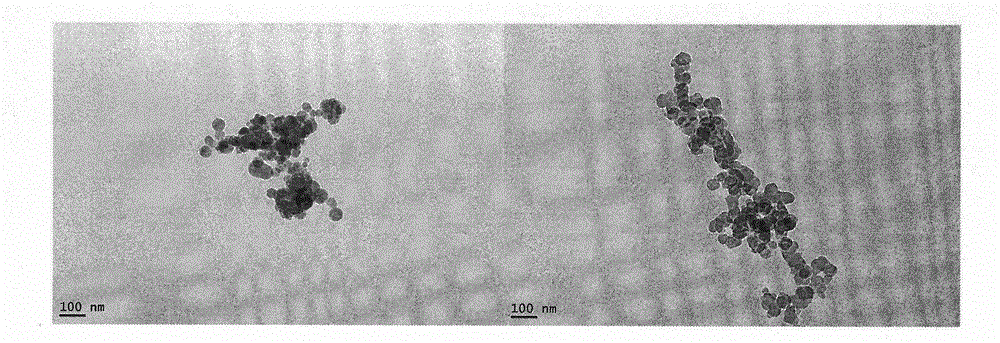
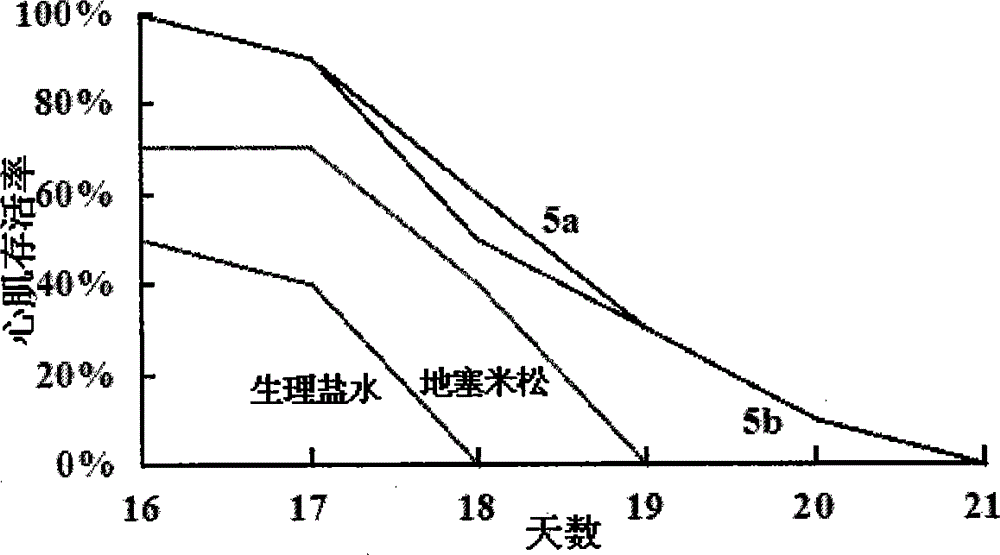
Octapeptide modified dexamethasone, preparation, nano-structure and application thereof
A technology of dexamethasone and -his-gly-glu, which is applied in the field of biomedicine and can solve the problems of no obvious improvement in curative effect and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Preparation of Boc-Gly-Glu(OBzl)-OBzl
[0027] Weigh (30.0mmol) Boc-Gly5.294g, 4.353g (32.5mmol) HOBt, dissolve in dry tetrahydrofuran (THF), stir under ice bath, add 7.406g (35.9mmol) DCC dissolved in THF dropwise, stir and activate for 30 Minutes later, 15.572g (31.3mmol) TosH·Glu(OBzl)-OBzl was added, adjusted to pH=8 by NMM, reacted at room temperature for 12 hours, TLC (CH 2 Cl 2 / MeOH=20 / 1), showing that the raw material point disappeared. DCU was removed by filtration, the filtrate was concentrated under reduced pressure, the residue was dissolved in 200 mL of ethyl acetate, and the obtained ethyl acetate solution was sequentially washed with 50 mL of saturated aqueous sodium bicarbonate, 50 mL of saturated aqueous sodium chloride, 50 mL of saturated aqueous potassium hydrogensulfate, and 50 mL of saturated aqueous chlorine Aqueous sodium chloride solution, 50 mL saturated aqueous sodium bicarbonate solution, and 50 mL saturated aqueous sodium chlorid...
Embodiment 2
[0028] Example 2 Preparation of HCl Gly-Glu(OBzl)-OBzl
[0029] Add 200mL4M hydrogen chloride ethyl acetate solution to 13.0g (26.9mmol) Boc-Gly-Glu(OBzl)-OBz under ice bath, after reacting for 2 hours, TLC (CH 2 Cl 2 / MeOH=20 / 1) showed disappearance of starting point. Concentrate under reduced pressure. The residue was dissolved in anhydrous ethyl acetate and concentrated under reduced pressure. This operation was repeated 3 times. The residue was dissolved in anhydrous ether and concentrated under reduced pressure. This operation was also repeated 3 times. Yield 12.5 g (96.1%) of the title compound.
Embodiment 3
[0030] Example 3 Preparation of Boc-His(Boc)-Gly-Glu(OBzl)-OBzl
[0031] Weigh 1.400g (3.9mmol) Boc-His (Boc), 0.561g (4.0mmol) HOBt, dissolve in dry tetrahydrofuran (THF), stir under ice bath, add dropwise 0.975g (4.7mmol) DCC dissolved in THF, After stirring and activating for 30 minutes, add 2.0g (4.8mmol) HCl Gly-Glu(OBzl)-OBzl, adjust the pH to 8 with NMM, react at room temperature for 12 hours, TLC (CH2 Cl 2 / MeOH=20 / 1), showing that the raw material point disappeared. Remove DCU by filtration, concentrate the filtrate under reduced pressure, dissolve the residue with 100mL ethyl acetate, and successively wash the obtained ethyl acetate solution with saturated sodium bicarbonate 20mL saturated aqueous sodium bicarbonate, saturated 20mL aqueous sodium chloride, 20mL saturated aqueous potassium bisulfate , 20mL saturated aqueous sodium chloride solution, 20mL saturated aqueous sodium bicarbonate solution, washed 3 times with 20mL saturated aqueous sodium chloride solution...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com