Method for preparing polyisocyanate containing biuret structure with stable storage
A polyisocyanate, storage-stable technology, applied in the field of polyisocyanate preparation, can solve the problems of surrounding environment and construction personnel injury, high cost of aldoxime compounds, limited application and other problems, and achieve the effect of excellent long-term storage stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
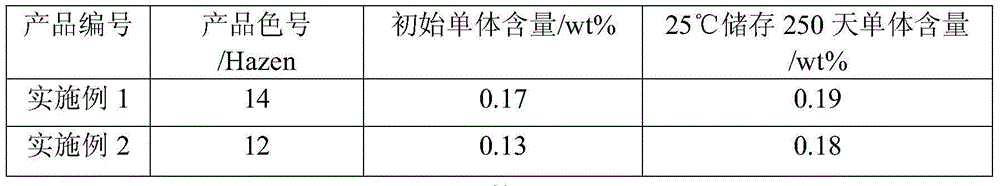
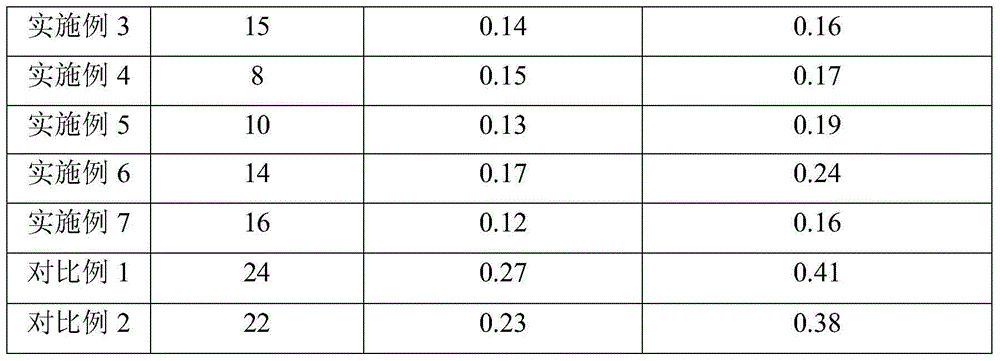
Examples
Embodiment 1
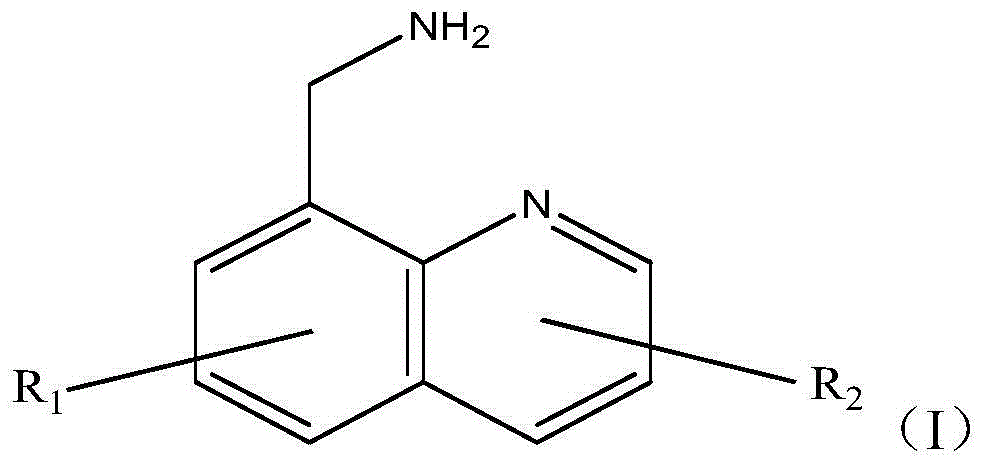
[0035]1) Dissolve 1.8g of 5-methyl-8-aminomethylquinoline and 2.86g of oxalic acid dihydrazide in 10.0g of water, preheat to 40°C, keep warm, and set aside;
[0036] 2) Under the protection of nitrogen, add 500g of hexamethylene diisocyanate (HDI) and 0.5g of diisopropyl phosphate into the reaction vessel, heat to 160°C, the dissolved 5-methyl-8-ammonia Add the water of methylquinoline and oxalic acid dihydrazide dropwise into the reaction vessel, and the dropping time is controlled at 50 minutes; after the dropwise addition, continue to stir and react at 160°C for 1 hour; then cool to below 35°C , blowing to obtain a biuret reaction solution;
[0037] 3) Enter the biuret reaction solution synthesized in step 2) into the secondary short-path evaporator separation device at a feed rate of 25mL / min to separate unreacted diisocyanate and polyisocyanate containing biuret structure, the first stage The separation temperature of the short-path evaporator is controlled at 140°C, the...
Embodiment 2
[0039] 1) Dissolve 1.02g of 5-chloro-8-aminomethylquinoline and 0.98g of adipic dihydrazide in 10.0g of water, preheat to 40°C, keep warm, and set aside;
[0040] 2) Under nitrogen protection, add 500g of hexamethylene diisocyanate (HDI) and 0.5g of diisopropyl phosphate into the reaction vessel, heat to 160°C, and dissolve 5-chloro-8-aminomethyl The water of quinoline and adipic acid dihydrazide was added dropwise into the reaction vessel, and the dropping time was controlled to be 50 minutes; after the dropwise addition, the stirring reaction was continued at 160°C for 1 hour; then cooled to below 35°C, and put The material obtains the biuret reaction liquid;
[0041] 3) Enter the biuret reaction solution synthesized in step 2) into the secondary short-path evaporator separation device at a feed rate of 25mL / min to separate unreacted diisocyanate and polyisocyanate containing biuret structure. The separation conditions are the same as Example 1: Dilute the polyisocyanate co...
Embodiment 3
[0043] 1) Dissolve 0.81g of 5-chloro-8-aminomethylquinoline and 0.07g of maleic hydrazide in 15.0g of water, preheat to 80°C, keep warm, and set aside;
[0044] 2) Under the protection of nitrogen, add 500g of hexamethylene diisocyanate (HDI) and 0.5g of diisopropyl phosphate into the reaction vessel, heat to 150°C, and dissolve the 5-chloro-8-aminomethyl The water of quinoline and maleic hydrazide was added dropwise into the reaction vessel, and the dropping time was controlled to be 60 minutes; after the dropping was completed, the stirring reaction was continued at 150°C for 1.5 hours; then cooled to below 35°C, and the material was discharged Obtain biuret reaction solution;
[0045] 3) Put the biuret reaction solution synthesized in step 2) into the secondary short-path evaporator separation device at a feed rate of 20mL / min to separate unreacted diisocyanate and polyisocyanate containing biuret structure. The separation conditions are the same as Example 1: Dilute the p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com