2,4-dichlorophenoxyacetic acid preparation method
A technology of dichlorophenoxyacetic acid and phenoxyacetic acid, which is applied in the preparation of carboxylate salts, organic compounds, chemical instruments and methods, etc., can solve the problems of many by-products and complicated processes, and simplify the cumbersome end point control Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
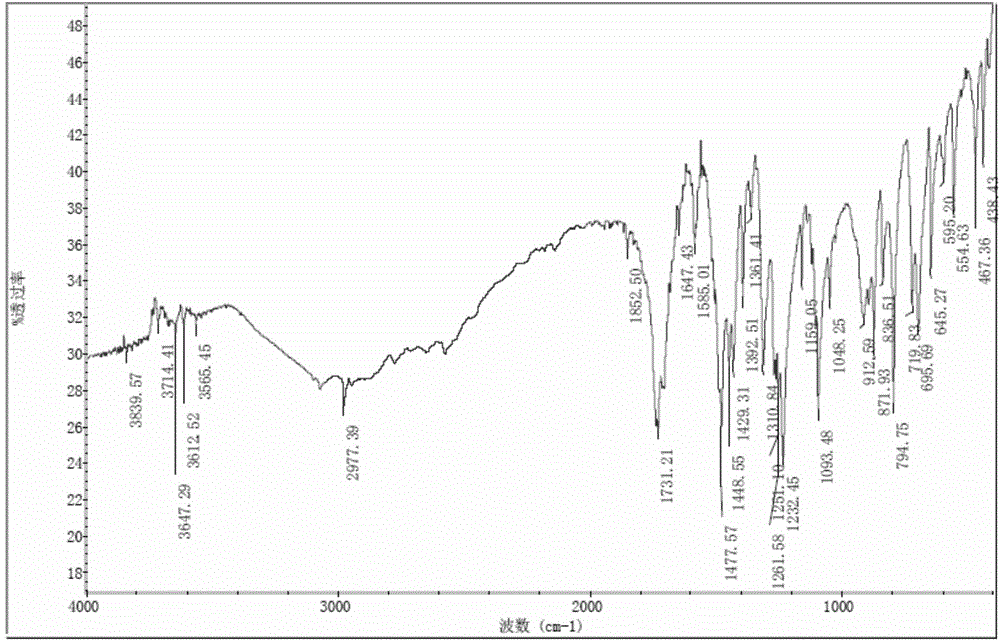
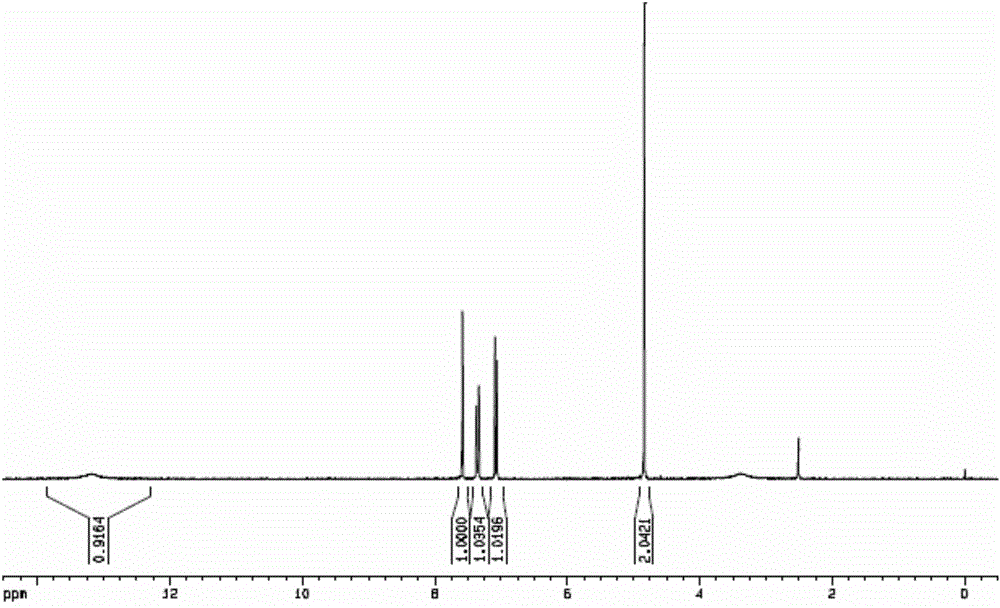
Image
Examples
preparation example Construction
[0035] The invention provides a kind of preparation method of 2,4-dichlorophenoxyacetic acid, comprises the following steps:
[0036] A) providing phenoxyacetic acid;
[0037] B) mixing hydrochloric acid, an oxidizing agent and the phenoxyacetic acid in the step A), and performing a chlorination reaction in the presence of a chlorination catalyst to obtain 2,4-dichlorophenoxyacetic acid;
[0038] The molar ratio of the phenoxyacetic acid, hydrochloric acid and oxidizing agent is 1:(2-200):(2-200), and the molar ratio of the hydrochloric acid and oxidizing agent is 1:(0.1-10).
[0039] The preparation method of 2,4-dichlorophenoxyacetic acid provided by the invention only needs one-step chlorination reaction to obtain 2,4-dichlorophenoxyacetic acid, and the purity and purity of 2,4-dichlorophenoxyacetic acid are guaranteed While improving the yield, the production process of 2,4-dichlorophenoxyacetic acid is simplified.
[0040] In the present invention, the phenoxyacetic aci...
Embodiment 1
[0079] In a 250ml four-necked flask, add 5g phenol, 5.1g chloroacetic acid and 10g water respectively, add liquid caustic soda to adjust the pH value to 12, add 0.5g catalyst (polyethylene glycol 400, benzyltriethylammonium chloride, cyclic The molar ratio of dextrin and tetrabutylphosphine acetate is: 10:5:2:1.5), using temperature program, the reaction solution rises from the initial 35°C to reflux in 3 minutes, and reflux reaction for 5 hours; cool down to room temperature, add hydrochloric acid Adjust the pH value to 2, cool to 10°C, crystallize for 3 hours, filter, wash, and dry at 90°C for 2 hours to obtain 7.49g of white needle-like crystals with a phenoxyacetic acid content of 98.2%; filtrate 13.1g, The phenoxyacetic acid content is 2.67%. The solid yield of phenoxyacetic acid was 91.9%, and the total yield was 97.95% (all calculated as phenol).
Embodiment 2
[0081] In a 250mL four-neck flask, add 5g phenol, 5.5g chloroacetic acid and 10g water respectively, add liquid caustic soda to adjust the pH value to 12, add 0.5kg catalyst (polyethylene glycol 800, dodecyltrimethylammonium chloride , 18 crown ethers 6, and the mol ratio of tetrabutylphosphine chloride are: 40:10:5:3), using a temperature program, rising from normal temperature to reflux within 3.5min, and reflux reaction for 3hr; cooling to room temperature, adding Adjust the pH value to 2 with hydrochloric acid, cool to 10°C, crystallize for 3 hours, filter, wash, and dry at 90°C for 2 hours to obtain 7.53g of white solid with a phenoxyacetic acid content of 99.1%; filtrate 13.4g, benzene The oxyacetic acid content is 3.50%. The solid yield of phenoxyacetic acid was 93.27%, and the total yield was 99.2% (all calculated as phenol).
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com