Method for serum-free culturing of 293T cells for lentivirus package
A technology of serum-free culture and lentivirus packaging, which is applied in the field of serum-free culture of 293T cells, and can solve the problem of mixing bovine serum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
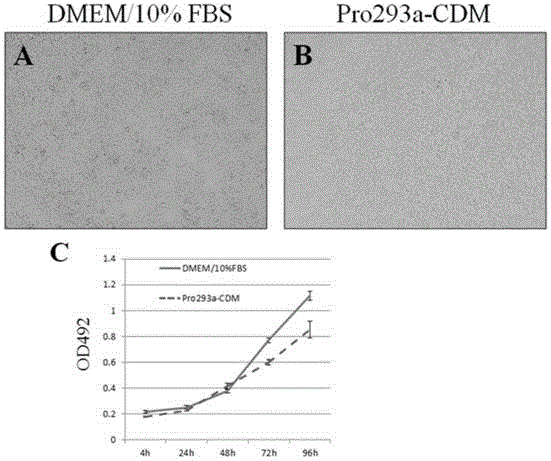
[0030] Step S1: Using DMEM medium containing 10% (volume ratio) fetal bovine serum, 293T cells were placed at 37°C, 5% CO 2 cultured in the incubator until the cells became confluent.
[0031] Step S2: use CDM medium containing 5% (volume ratio) fetal bovine serum (in step 2, replace the medium with Pro293a-CDM, referred to as CDM medium, which is a serum-free 293 cell medium, LONZA Company, product number: 12-764Q), place the 293T cells cultured in step S1 at 37°C, 5% CO 2 cultured in the incubator until the cells became confluent. At this time, the cell morphology and proliferation rate did not change significantly.
[0032] In an orderly manner, subculture is carried out after the cells are overgrown, and after 5 consecutive passages (about 10-12 days for 5 passages), some cells are sampled for cryopreservation.
[0033] Step S3, use CDM medium containing 2% (volume ratio) fetal bovine serum, and place the 293T cells cultured in step S2 at 37°C in 5% CO. 2 cultured in t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com