Prostate-cancer-specific PAP (prostatic acid phosphatase)-GM-CSF (granulocyte-macrophage colony-stimulating factor)-IL-6 (interluekin-6) genetic recombinant fusion protein and preparation method thereof
A PAP-GM-CSF-IL-6, fusion protein technology, applied in biochemical equipment and methods, fusion polypeptides, drug combinations, etc., can solve the problems of low targeting, poor specificity, and low loading efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] The plasmid pcDNA3.1 used in the present invention was purchased from Biovector; the HEK293 cell line was purchased from the Cell Bank of the Chinese Academy of Sciences; the plasmid extraction kit and gel recovery kit were purchased from Tiangen Biochemical Technology Co., Ltd.; NiSepharoseTM6FastFlow was purchased from QIAGEN.
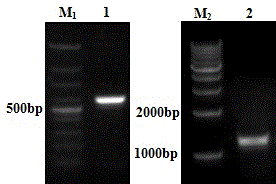
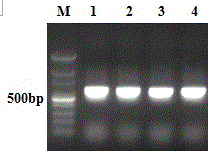
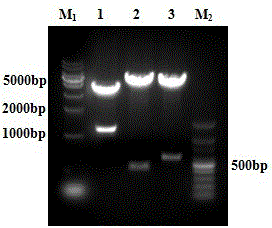
[0063] 1.1 Construction of pcDNA3.1-PAP-GM-CSF-IL-6 fusion protein expression plasmid
[0064] 1.1.1 Design and synthesis of primers:
[0065] According to the restriction sites of pcDNA3.1 plasmid and the PAP and IL-6 gene sequences provided by NCBI, the upstream and downstream primers of PAP and IL-6 genes were designed respectively. The sequence of the upstream primer of PAP is: 5'CTAGCTAGCCGGCTCTCCTCAACATGAG3'; the sequence of the downstream primer is: 5'GCCGCTCGAGATCTGTACTGTCCTCAGT3'; the upstream and downstream primers respectively introduce restriction endonuclease NheI and XhoI enzyme cutting sites. The upstream primer sequence of IL-...
Embodiment 2
[0090] The invention provides a DC vaccine prepared by PAP-GM-CSF-IL-6 gene recombinant protein, which has the antigenic characteristics of PAP and the immune function of GM-CSF and IL-6, and can be applied to the therapeutic DC vaccine. Preparation, as well as the preparation of prostate cancer-specific CTL effector cells, thus, is the tumor-specific antigen of choice in cellular immunotherapy. The therapeutic DC vaccine prepared by using the gene recombinant protein has high-efficiency T cell activation effect and can effectively improve clinical curative effect. The steps are as follows:
[0091] (1) Obtaining peripheral blood mononuclear cells: extract 100ml of peripheral blood from healthy people, and obtain monocytes by gradient density centrifugation;
[0092] (2) After culturing in serum-free medium for 2 hours, the suspended cells were harvested to be expanded and cultured as T cells, and the adherent cells were activated and cultured as DC cells, which were cultured...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com