Application of tranilast in preparation of drug for treating pyoderma gangrenosum
A tranilast and diabetes-based technology, which can be used in drug combinations and skin diseases, and can solve the problems of lack of specific and effective treatments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0011] The following shows that tranilast can be used as a drug for treating diabetic scleredema through the specific clinical application of tranilast in the treatment of diabetic scleredema.
[0012] The used Tranister (trade name "Qukeshen", China Pharmaceutical University Pharmaceutical Co., Ltd.) has a specification of 100 mg per tablet.
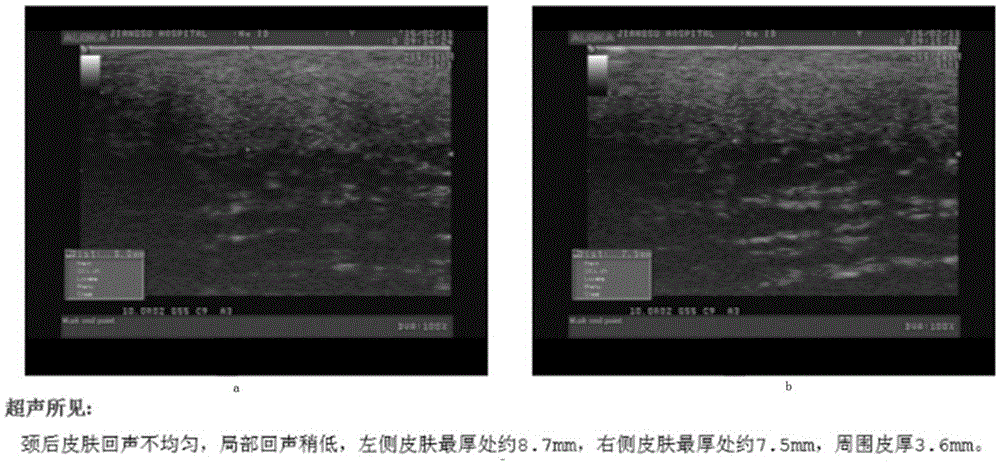
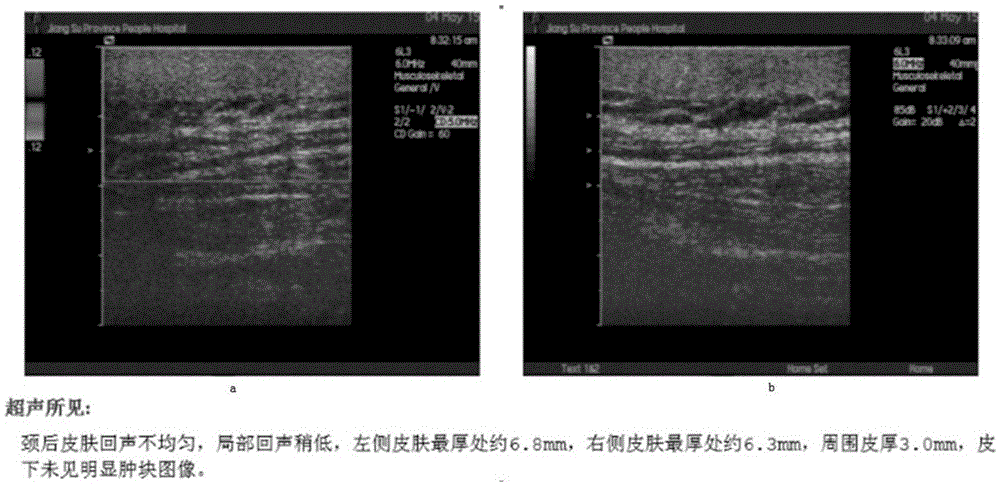
[0013] Among the 3 patients, 2 were males in their 40s, and 1 was a female in their 60s. Both had suffered from type 2 diabetes for many years, and the medical history of diabetic scleredema was more than 5 years. B-ultrasound examination showed that the skin thickness increased to varying degrees. Tranilast Capsules 0.1 was given orally 3 times a day. After 3 months, the local skin erythema obviously subsided, became thinner, and became softer. figure 1 It is a B-ultrasound picture of a male patient in his 40s before taking the medicine (a and b are different parts of the affected skin). The B-ultrasound test found that the skin thickn...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com