2-substituted benzopyran-4-one compounds and application thereof
A kind of technology of benzopyran, compound, be used in the field of 2-substituted benzopyran-4-one compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 15
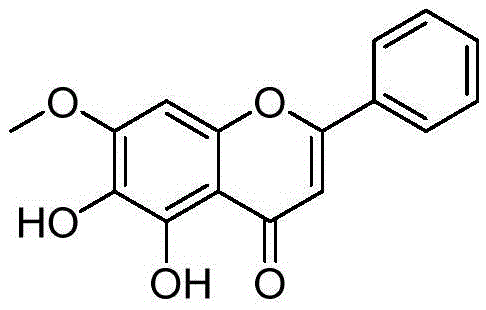
[0146] Example 15, Preparation of 6-dihydroxy-7-methoxy-2-phenyl-4H-benzopyran-4-one (compound 1 in the table)
[0147]
[0148] Take baicalein 100mg (0.37mmol), join in 15mlDMF, then add CH 3 I105mg (0.74mmol), K 2 CO 3 153mg. After stirring and reacting at room temperature for 12 hours, the reaction solution was poured into ice water, filtered, the solid was washed with water three times, dried and separated by flash column to obtain 43 mg of compound (1) with a yield of 31%. M+ = 285.1.
Embodiment 25
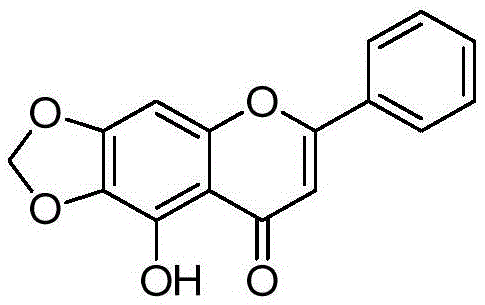
[0149] Preparation of Example 25-hydroxyl-6,7-methylenedioxy-2-phenyl-4H-benzopyran-4-one (compound 3 in the table)
[0150]
[0151] Take baicalein 100mg (0.37mmol), add it to anhydrous DMF15ml, then add BrCH 2 Cl52.1mg (0.41mmol), CsCO 3 150mg. Ar 2 After stirring and reacting for 12 hours at room temperature under protection, the reaction solution was poured into ice water, filtered, the solid was washed with water three times, dried and separated by flash column to obtain 80 mg of compound 6,7-methylenedioxy-5-hydroxyflavone, produced The rate is 77%. m + =283.0,M+Na + = 305.0.
Embodiment 36
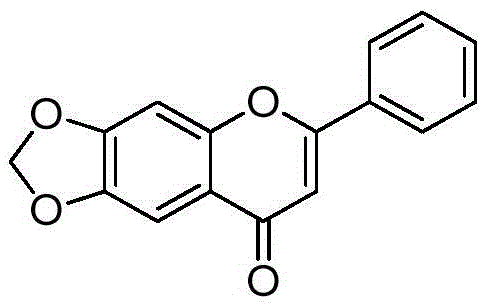
[0152] Example 36, Preparation of 7-methylenedioxy-2-phenyl-4H-benzopyran-4-one (compound 4 in the table)
[0153]
[0154] Synthesized according to the method in the literature (Bioorganic & Medicinal Chemistry, 15(23), 7408-7425; 2007). m + = 267.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com