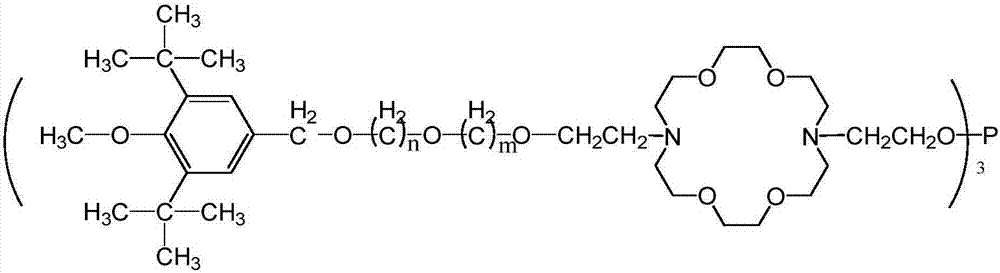
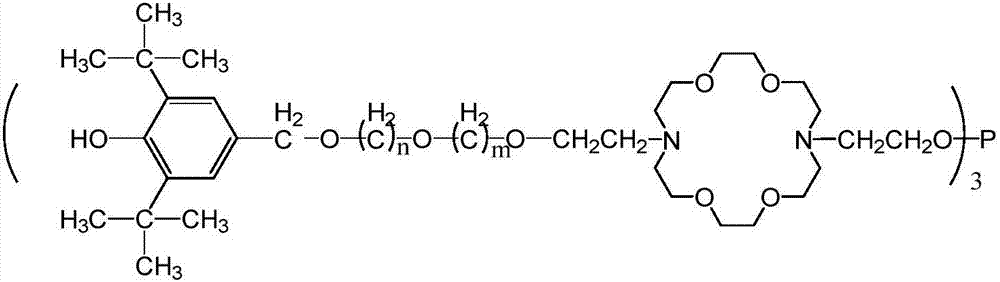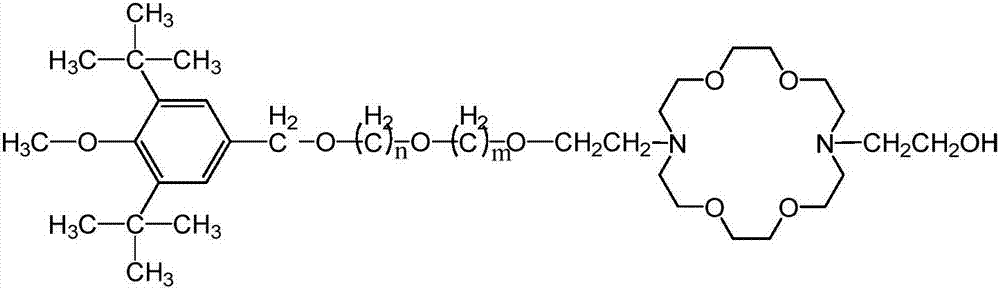A kind of hindered phenol and phosphite amphiphile compound and its synthesis method and application
A technology of amphiphile compounds and synthesis methods, applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc., can solve the problem of reducing the antioxidant effect and the synergy between phenols and phosphites. the manifestation of
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Under nitrogen protection, 220g of 2,6-di-tert-butyl-p-cresol and 150g of dimethyl sulfate were dissolved in 2.5L of dry chloroform. The reaction solution was heated to 60° C. under stirring in a 5 L three-necked flask, and the reaction continued for 1.5 hours. After the reaction solution was cooled to 25°C, it was repeatedly extracted four times with 1 L of 0.5 mol / L potassium carbonate solution, and the organic layer was dried with anhydrous magnesium sulfate for 24 hours. The anhydrous magnesium sulfate desiccant in the solution was removed by filtration, and the solvent was distilled off under reduced pressure to obtain 215.3 g of 2,6-di-tert-butyl-p-methylanisole, with a yield of 92%.
[0091] Under nitrogen protection, 215.3g of 2,6-di-tert-butyl-p-methylanisole was added into a 5L three-necked flask containing 3L of carbon tetrachloride. Then add 161g of N-bromosuccinimide and 2g of azobisisobutyronitrile, heat the reaction solution to 77°C for 3 hours, stop hea...
Embodiment 2
[0098] Under nitrogen protection, 220g of 2,6-di-tert-butyl-p-cresol and 150g of dimethyl sulfate were dissolved in 2.5L of dry chloroform. The reaction solution was heated to 60° C. under stirring in a 5 L three-necked flask, and the reaction continued for 1.5 hours. After the reaction solution was cooled to 25°C, it was repeatedly extracted four times with 1 L of 0.5 mol / L potassium carbonate solution, and the organic layer was dried with anhydrous magnesium sulfate for 24 hours. The anhydrous magnesium sulfate desiccant in the solution was removed by filtration, and the solvent was evaporated under pressure to obtain 215.3 g of 2,6-di-tert-butyl-p-methylanisole, with a yield of 92%.
[0099] Under nitrogen protection, 215.3g of 2,6-di-tert-butyl-p-methylanisole was added into a 5L three-necked flask containing 3L of carbon tetrachloride. Then add 161g of N-bromosuccinimide and 2g of azobisisobutyronitrile, heat the reaction solution to 77°C for 3 hours, stop heating, cool ...
Embodiment 3
[0106] Under nitrogen protection, 220g of 2,6-di-tert-butyl-p-cresol and 150g of dimethyl sulfate were dissolved in 2.5L of dry chloroform. The reaction solution was heated to 60° C. under stirring in a 5 L three-necked flask, and the reaction continued for 1.5 hours. The reaction solution was cooled to 25°C and extracted four times with 1LO.5 mol / L potassium carbonate solution, and the organic layer was dried with anhydrous magnesium sulfate for 24 hours. The anhydrous magnesium sulfate desiccant in the solution was removed by filtration, and the solvent was distilled off under reduced pressure to obtain 215.3 g of 2,6-di-tert-butyl-p-methylanisole, with a yield of 92%.
[0107] Under nitrogen protection, 215.3g of 2,6-di-tert-butyl-p-methylanisole was added into a 5L three-necked flask containing 3L of carbon tetrachloride. Then add 161g of N-bromosuccinimide and 2g of azobisisobutyronitrile, heat the reaction solution to 77°C for 3 hours, stop heating, cool the reaction so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com