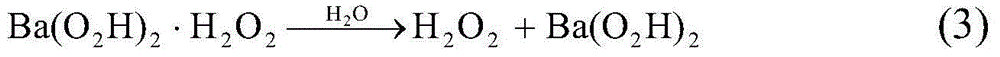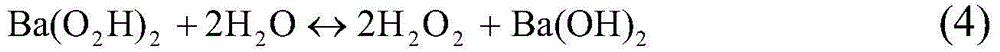Method of using hydrogen peroxide sustained releasing agent and chlorine in combination for fuel oil oxidation
A hydrogen peroxide, oxidation treatment technology, applied in the direction of refining with oxygenated compounds, can solve the problems of increasing the cost of fuel oil oxidation treatment, prone to explosive decomposition, hidden dangers in storage and transportation, etc., to reduce the cost of oxidation treatment, easy to obtain , the effect of simple equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Simulated gasoline formulated from n-octane and 4,6-dimethyldibenzothiophene (composed of 3.3112g 4,6-dimethyldibenzothiophene + 996.7g n-octane) and decahydronaphthalene and 4 , The simulated diesel oil formulated with 6-dimethyldibenzothiophene (composed of 3.3112g4,6-dimethyldibenzothiophene+996.7g decahydronaphthalene) had a sulfur content of 500mg / kg. Take 50ml of any simulated fuel (simulated gasoline or simulated diesel) and keep the simulated fuel at 0→C. Add 1.4gMgO to the simulated fuel successively2 solid (or 1.8gCaO 2 solid, or 2.9gSrO 2 solid, or 4.1gBaO 2 solid, or 2.6gCa(O 2 ) 2 solid, or 2.7gSr(O 2 ) 2 solid, or 4.9gBa(O 2 ) 2 solid) and 3.4ml of water, stirred to mix the oil phase and the water phase, and chlorine gas was passed into the simulated fuel oil at a chlorine gas flow rate of 0.065mmol / min, and the chlorine gas was stopped after 5 hours to complete the reaction, and the reaction mixture was separated after standing. Separate the wate...
Embodiment 2
[0036] Simulated gasoline formulated from n-octane and 4,6-dimethyldibenzothiophene (composed of 3.3112g 4,6-dimethyldibenzothiophene + 996.7g n-octane) and decahydronaphthalene and 4 , The simulated diesel oil formulated with 6-dimethyldibenzothiophene (composed of 3.3112g4,6-dimethyldibenzothiophene+996.7g decahydronaphthalene) had a sulfur content of 500mg / kg. Take 50ml of any simulated fuel (simulated gasoline or simulated diesel) and keep the simulated fuel at 0→C. Add 3.8gBa(O 2 h) 2 Solid (or 2.9gSr(O 2 h) 2 solid, or 4.4gBa(O 2 h) 2 ·H 2 o 2 solid, or 3.5gSr(O 2 h) 2 ·H 2 o 2 solid) and 3.3ml of water, stirred to mix the oil phase and the water phase, and chlorine gas was passed into the simulated fuel oil at a chlorine gas flow rate of 0.026mmol / min, and the chlorine gas was stopped after 5 hours to complete the reaction, and the reaction mixture was separated after standing. Separate the water layer and the oil layer, use 50ml of water to extract the sulf...
Embodiment 3
[0038] Simulated gasoline formulated from n-octane and 4,6-dimethyldibenzothiophene (composed of 3.3112g 4,6-dimethyldibenzothiophene + 996.7g n-octane) and decahydronaphthalene and 4 , The simulated diesel oil formulated with 6-dimethyldibenzothiophene (composed of 3.3112g4,6-dimethyldibenzothiophene+996.7g decahydronaphthalene) had a sulfur content of 500mg / kg. Take 50ml of any simulated fuel (simulated gasoline or simulated diesel) and keep the simulated fuel at 0→C. Add 5.8g2Na to the simulated fuel successively 2 CO 3 ·3H 2 o 2 Stir the solid and 3.3ml of water to mix the oil phase and the water phase, and inject chlorine gas into the simulated fuel oil at a chlorine gas flow rate of 0.056mmol / min. Stop feeding chlorine gas after 5 hours to complete the reaction, and the reaction mixture is separated after standing , the water layer and the oil layer were separated, the sulfur in the oil layer was extracted with 50ml of water for the first time, and then 50ml of N,N-d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com