Use of chromone derivatives as dopamine d3 receptor antagonists for the treatment of autism spectrum disorders
A use, chromene technology, applied in the field of use of chromone derivatives as dopamine D3 receptor antagonists for the treatment of autism spectrum disorders, can solve the problem of not teaching the treatment of ASD and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
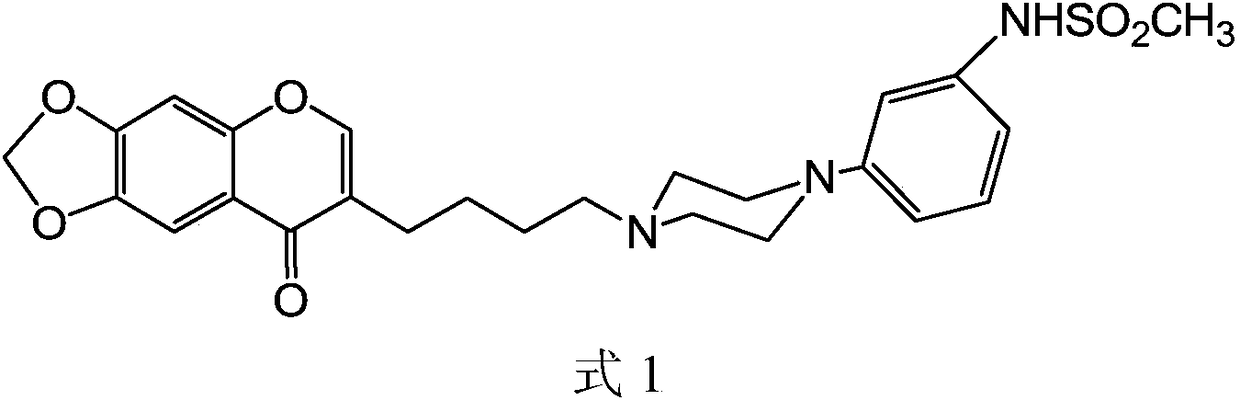
[0047] According to the present invention, N-(3-{4-[4-(8-oxo-8H-[1,3] Dioxole[4,5-g]chromen-7-yl)-butyl]-piperazin-1-yl}-phenyl)-methanesulfonamide hydrochloride as dopamine D3 receptor ligands and modulators of this receptor activity. Through [ 3 H] Inhibition of Spiperone Binding Inhibition constants (Ki) were determined. The inventors have demonstrated that the compounds according to the invention behave as potent dopamine D3 receptor ligands with 0.17 nmol l -1 The Ki value. This same compound showed a significant 71-fold attenuation of affinity for the dopamine D2 receptor.
[0048]The agonist, partial agonist or antagonist activity of the compounds according to the invention on the dopamine D3 receptor is evaluated by using the MAP-kinase activity assay on the human recombinant dopamine D3 receptor (Cussac et al., Mol.Pharmacol.1999, vol. 56, p1025-1030). The intrinsic activity of this compound is zero, indicating that it is a full antagonist.
Embodiment 2
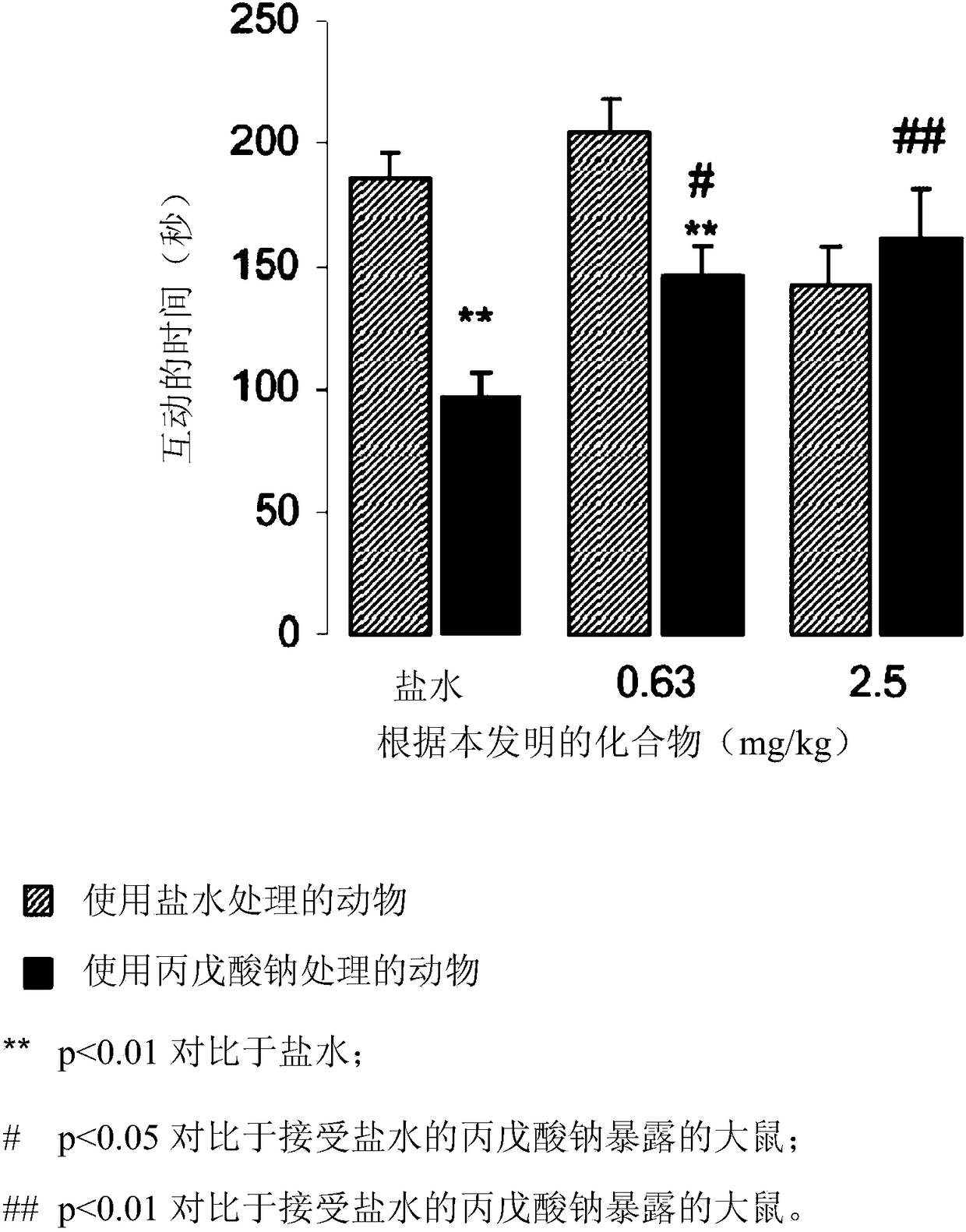
[0050] N-(3-{4-[4-(8-oxo-8H-[1,3]dioxole[4,5-g] Chrom-7-yl)-butyl]-piperazin-1-yl}-phenyl)-methanesulfonamide hydrochloride, the female rats had been administered the sodium salt of valproic acid. The experimental setup of the valproic acid autism rat model was adapted from published data (Dendrinos et al., Front. Integr. Neurosci. 2011, vol 5, art 68; Markram et al., Neuropsychopharm. -912; Schneider et al., Neuropsychopharm. 2005, vol30, p 80-89).
[0051] method:
[0052] Pregnant (maximum gestational age 8 days) female Sprague-Dürer rats [OFA (SD) Charles River Lyon, France] were isolated for 4 days. In an environmentally controlled room (temperature 21±1°C; relative humidity 55±5%), under a 12h light / dark cycle (lights turned on at 07:00 am), in full-bottomed cages (ML-H cages, 370x235x180mm ,LxWxH; floor area 870cm 2 ) in group housing (2 animals per cage) with free access to food (A04, Safe, Augy, France) and filtered water (0.2 μm pore size). Until the offspring...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com