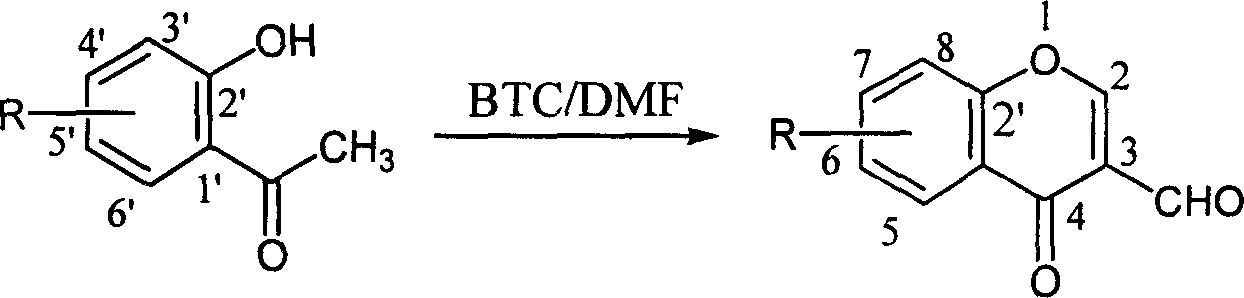Method for preparing 3-formacyl chromone derivative
A technology for formyl chromone and derivatives, which is applied in the field of preparation of 3-formyl chromone derivatives, can solve the problems of large change in yield, many reaction steps, difficult preparation of raw materials and the like, and achieves high reaction yield, The effect of improved reaction yield and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The preparation of embodiment 1 3-formyl chromone
[0021] Add 23.0mL of N,N-dimethylformamide (300mmol) and 20mL of 1,2-dichloroethane into a 250mL four-necked reaction flask equipped with mechanical stirring, a drying tube, a thermometer, and a dropping funnel. Stir and cool down to 0-5°C, then add bis(trichloromethyl)carbonate solution (18g, 60mmol, dissolved in 50mL 1,2-dichloroethane) dropwise, and react at 0-5°C for one hour, then dropwise add o-hydroxyacetophenone solution (4.1g, 30mmol, dissolved in 10mL1,2-dichloroethane) at 0-5°C, and react for one hour at 0-5°C after dropping, and then The temperature was raised to 25-30° C. for 12 hours, and the progress of the reaction was tracked by TLC. After the reaction, the reaction product was separated and purified: the above reaction mixture was poured into 200g of crushed ice, stirred for 1 to 1.5 hours for complete hydrolysis, then the organic layer was separated, and the aqueous layer was extracted with 1,2-dich...
Embodiment 2
[0023] The preparation of embodiment 2 3-formyl chromone
[0024]Add 23.0mL of N,N-dimethylformamide (300mmol) and 20mL of dichloromethane into a 250mL four-necked reaction flask equipped with a mechanical stirrer, a drying tube, a thermometer, and a dropping funnel, stir and cool to 0mL under an ice-water bath -5°C, then add bis(trichloromethyl)carbonate solution (18g, dissolved in 50mL dichloromethane) dropwise, react at 0-5°C for one hour after dropping, then add dropwise at 0-5°C o-Hydroxyacetophenone solution (4.1g, 30mmol, dissolved in 10mL dichloromethane), reacted at 0-5°C for one hour after dropping, then raised the temperature to 25-30°C for 12 hours, followed the progress of the reaction by TLC. The separation and purification steps were the same as in Example 1 to obtain 3.76 g of 3-formylchromone, with a yield of 72%, and the physical properties were the same as in Example 1.
Embodiment 3
[0025] Example 3 Preparation of 3-formylchromone
[0026] Add 27.6mL of N,N-dimethylformamide (360mmol) and 20mL of dichloromethane into a 250mL four-necked reaction flask equipped with a mechanical stirrer, a drying tube, a thermometer and a dropping funnel, stir and cool to 0mL under an ice-water bath -5°C, then add bis(trichloromethyl)carbonate solution (18g, 60mmol, dissolved in 50mL dichloromethane) dropwise, and react at 0-5°C for one hour after dropping, and then at 0-5°C Add o-hydroxyacetophenone solution (4.1g, 30mmol, dissolved in 10mL dichloromethane) dropwise, react at 0-5°C for one hour after dropping, then raise the temperature to 25-27°C for 12 hours, track the progress of the reaction by TLC . The separation and purification steps were the same as in Example 1 to obtain 4.5 g of 3-formylchromone with a yield of 86%, and the physical properties were the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com