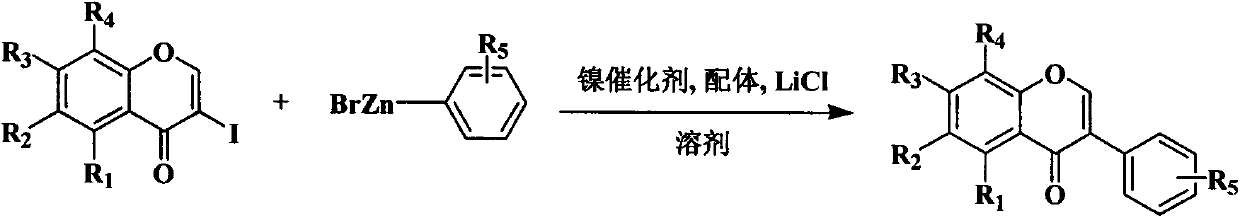
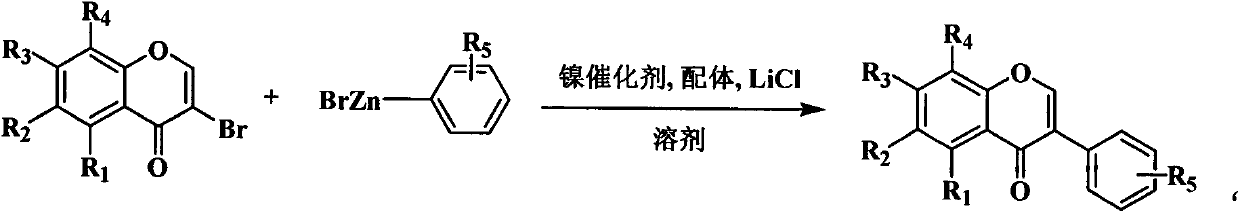
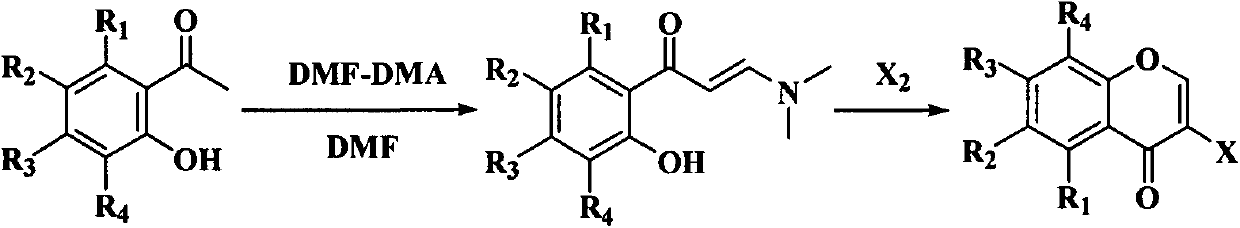
Method for synthesizing isoflavone by nickel-catalyzed Negishi cross coupling reaction at room temperature
A technology of isoflavones and compounds, applied in the field of heterocyclic compounds, can solve the problems of complex reaction process, high price, and reduced catalytic efficiency, and achieve the effects of simple raw material preparation, mild reaction conditions, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] In this embodiment, 3-iodochromanone and tetrahydrofuran with 15 times the weight of 3-iodochromanone were added to the reaction kettle, anhydrous nickel chloride with 0.03 times the molar amount of 3-iodochromanone, and 3-iodochromogen Triphenylphosphine of 0.06 times the molar quantity of ketone, anhydrous lithium chloride of 1.5 times the molar quantity of 3-iodochromanone, after stirring evenly, add phenylzinc bromide of 1.1 times the molar quantity of 3-iodochromanone, After reacting at room temperature for 1 hour, add 3 times the weight of 3-iodochromanone in 1mol / L hydrochloric acid aqueous solution to quench the reaction, then add 30 times the weight of 3-iodochromanone distilled water, and use distilled water equal volume ethyl acetate Extract the ester three times, combine the organic phases, wash the organic phases with distilled water until neutral, dry over anhydrous magnesium sulfate, collect the ethyl acetate layer, recover the solvent under reduced pressu...
Embodiment 2
[0057] In this example, 3-iodochromanone and acetonitrile with 20 times the weight of 3-iodochromanone, anhydrous nickel chloride with 0.05 times the molar amount of 3-iodochromanone, and 3-iodochromanone were added to the reaction kettle. Tris (2-furyl) phosphine with 0.1 times the molar amount of the original ketone mole, anhydrous lithium chloride with 1.5 times the molar amount of 3-iodochromanone, after stirring evenly, add 1.2 times the molar amount of 3-iodochromanone respectively Phenyl zinc bromide, 4-methylphenyl zinc bromide, 2-methoxyphenyl zinc bromide, 3-methoxyphenyl zinc bromide, 4-methoxyphenyl zinc bromide, 2-cyanophenyl zinc bromide, 3-cyanophenyl zinc bromide, 4-cyanophenyl zinc bromide, 4-trifluoromethylphenyl zinc bromide, 4-chlorophenyl zinc bromide Zinc and 4-benzoic acid methyl zinc bromide stop the reaction after reacting at room temperature for 2 hours, and the separation process and method of the product are the same as the preparation of isoflavone...
Embodiment 3
[0149]In this example, 6-fluoro-3-iodochromanone and acetonitrile with 30 times the weight of 6-fluoro-3-iodochromanone were added to the reaction kettle, and 0.02 times the mole of 6-fluoro-3-iodochromanone The amount of anhydrous nickel chloride, 0.04 times the molar amount of 6-fluoro-3-iodochromanone tris (4-methylphenyl) phosphine, 1.5 times the molar amount of 6-fluoro-3-iodochromanone without Lithium chloride in water, after stirring evenly, add phenyl zinc bromide, 4-methylphenyl zinc bromide, 4-methoxyphenyl Zinc chloride, 2-cyanophenyl zinc bromide, 4-trifluoromethylphenyl zinc bromide and 4-chlorophenyl zinc bromide stop the reaction after reacting at room temperature for 3 hours, and the separation process and method of the product Same as the preparation of the isoflavone compound in Example 1, the compound (12) 6-fluoroisoflavone, the compound (13) 6-fluoro-4′-methyl isoflavone, the compound (14) 6-fluoro-4′ were obtained respectively -Methoxyisoflavone, compoun...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com