Synthetic method of 2-alkyl substituted chromanone compound
A synthesis method and compound technology, applied in organic chemistry and other directions, can solve the problems of unobtainable raw materials, cumbersome reaction operations, and waste generation, and achieve the effects of high alkyl diversity, high reaction yield, and wide sources.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
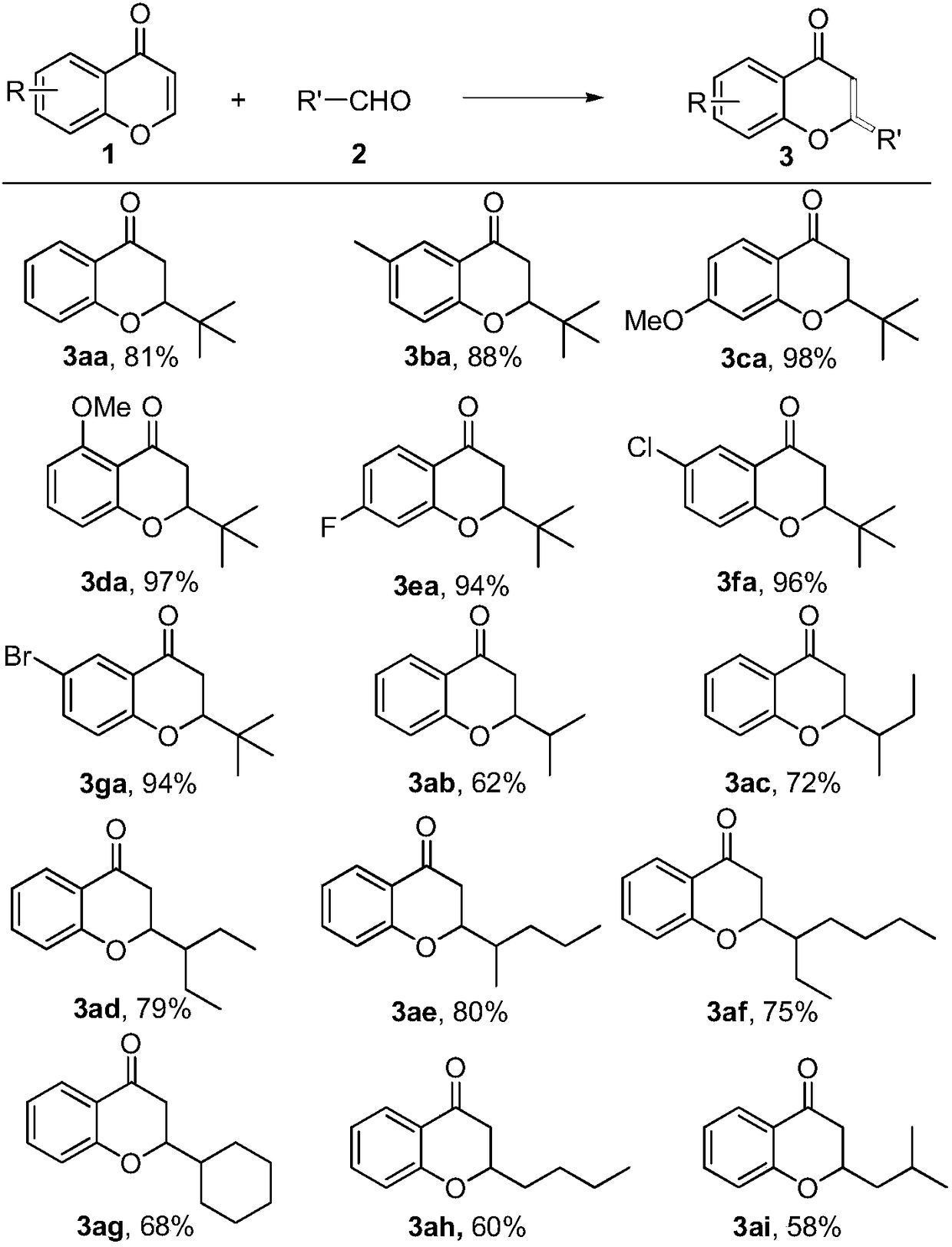
[0014] Under nitrogen protection, chromone 1a (0.2 mmol), pivalaldehyde 2a (0.8 mmol), di-tert-butyl peroxide (DTBP, 0.6 mmol), and isopropanol (1 mL) were added to a Schlenk reaction tube and sealed. Heating to 120°C, the reaction time was 12 hours. After the reaction, the solvent was removed under reduced pressure, and the target product 3aa was obtained through column chromatography. The conversion of chromone 1a was 94%, and the yield of 2-tert-butylchromanone 3aa was 81%. 1 H NMR (CDCl 3 ,400MHz):δ7.85(dd,J=8.1,1.8Hz,1H),7.47-7.42(m,1H),6.99-6.95(m,2H),4.04(dd,J=12.6,4.1Hz,1H ), 2.72-2.61(m,2H), 1.05(s,9H).
Embodiment 2
[0016] Under nitrogen protection, 6-methylchromone 2b (0.2 mmol), pivalaldehyde 2a (0.8 mmol), tert-butyl hydroperoxide (TBHP, 0.6 mmol), isopropanol (1 mL) were added to a Schlenk reaction tube In, sealed. Heating to 120°C, the reaction time was 12 hours. After the reaction, the solvent was removed under reduced pressure, and the target product 3ba was obtained through column chromatography. The conversion of chromone 2b was 92%, and the yield of 6-methyl-2-tert-butylchromanone 3ba was 88%. 1 H NMR (CDCl3, 400MHz): δ7.61 (d, J = 1.6Hz, 1H), 7.23 (dd, J = 8.4, 2.2Hz, 1H), 6.84 (d, J = 8.4Hz, 1H), 4.03- 3.91 (m, 1H), 2.67-2.56 (m, 2H), 2.26 (s, 3H), 1.02 (s, 9H).
Embodiment 3
[0018] Under nitrogen protection, add 7-methoxychromone 2c (0.2 mmol), pivalaldehyde 2a (0.8 mmol), di-tert-butyl peroxide (DTBP, 0.6 mmol), isopropanol (1 mL) into a Schlenk reaction tube In, sealed. Heating to 120°C, the reaction time was 12 hours. After the reaction, the solvent was removed under reduced pressure, and the target product 3ca was obtained through column chromatography. The conversion of chromone 2c was 100%, and the yield of 2-tert-butyl-7-methoxychromanone 3ca was 98%. 1 H NMR (CDCl 3 ,400MHz): δ7.73(d,J=8.8Hz,1H),6.49(dd,J=8.8,2.2Hz,1H),6.36(d,J=2.3Hz,1H),4.00-3.96(m, 1H), 3.77(s, 3H), 2.62-2.48(m, 2H), 1.00(s, 9H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com