Boron material and preparation method and application thereof
A technology for electromechanical and phosphorescent devices, used in chemical instruments and methods, semiconductor/solid-state device manufacturing, electrical components, etc., to achieve the effect of increasing the molecular weight of compounds, high-efficiency electroluminescence performance, and good hole/electron balance ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
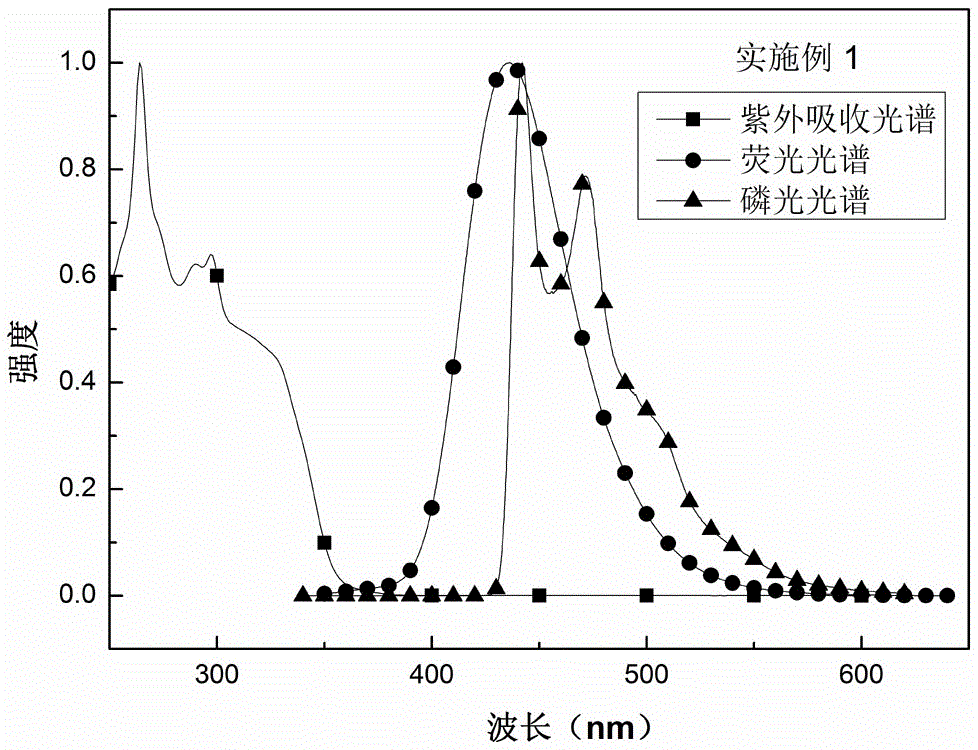
Embodiment 1
[0026] A preparation method of boron material, the steps of the method are as follows:
[0027] Step 1: Dissolve 3.00g of 3-bromospirofluorene ring-closed triphenylamine in anhydrous tetrahydrofuran under the protection of nitrogen, cool to -78°C, and then add 3.9mL of n-butyllithium dropwise. Reaction at ℃ for 1h;
[0028] The second step: add a tetrahydrofuran solution containing 2.49 g of bis(trimethylphenyl)boron fluoride to the reaction system of the first step, react at a temperature of -78°C for 2 hours, then raise the temperature to room temperature, and react at room temperature for 12 hours;
[0029] The third step: add water to the reaction system of the second step to quench the reaction, then wash with water, then extract the organic layer with dichloromethane, then dry the organic layer with anhydrous sodium sulfate, then spin dry, and use a volume ratio of 1 :5 dichloromethane / petroleum ether passes through the column, spins dry again, recrystallizes, obtains t...
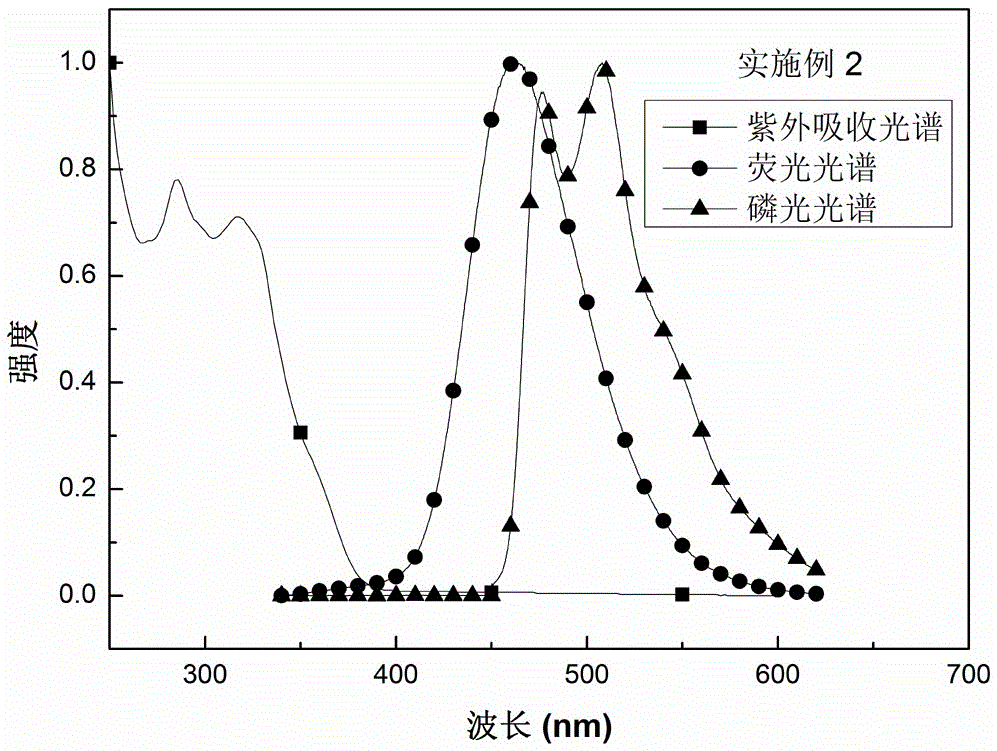
Embodiment 2
[0032] A preparation method of boron material, the steps of the method are as follows:
[0033] Step 1: Dissolve 4.00g of 4-bromospirofluorene ring-closed triphenylamine in anhydrous tetrahydrofuran under the protection of nitrogen, cool to -78°C, and then add 5.2mL of n-butyllithium dropwise. Reaction at ℃ for 1h;
[0034] The second step: add a tetrahydrofuran solution containing 3.32 g of bis(trimethylphenyl)boron fluoride to the reaction system of the first step, react at a temperature of -78°C for 2 hours, then raise the temperature to room temperature, and react at room temperature for 12 hours;
[0035] The third step: add water to the reaction system of the second step to quench the reaction, then wash with water, then extract the organic layer with dichloromethane, then dry the organic layer with anhydrous sodium sulfate, then spin dry, and use a volume ratio of 1 :5 dichloromethane / petroleum ether passes through the column, spins dry again, recrystallizes, obtains t...
Embodiment 3
[0038] A preparation method of boron material, the steps of the method are as follows:
[0039] Step 1: Dissolve 4.50g of 3-bromospirofluorene in anhydrous tetrahydrofuran under the protection of nitrogen, cool to -78°C, then add 5.7mL of n-butyllithium dropwise, and react at -78°C 1h;
[0040]The second step: add a tetrahydrofuran solution containing 3.98 g of bis(trimethylphenyl)boron fluoride to the reaction system of the first step, react at a temperature of -78°C for 2 hours, then raise the temperature to room temperature, and react at room temperature for 12 hours;
[0041] The third step: add water to the reaction system of the second step to quench the reaction, then wash with water, then extract the organic layer with dichloromethane, then dry the organic layer with anhydrous sodium sulfate, then spin dry, and use a volume ratio of 1 :5 dichloromethane / petroleum ether passes through the column, spins dry again, recrystallizes, obtains the SF-3-DMB boron material of 3...
PUM
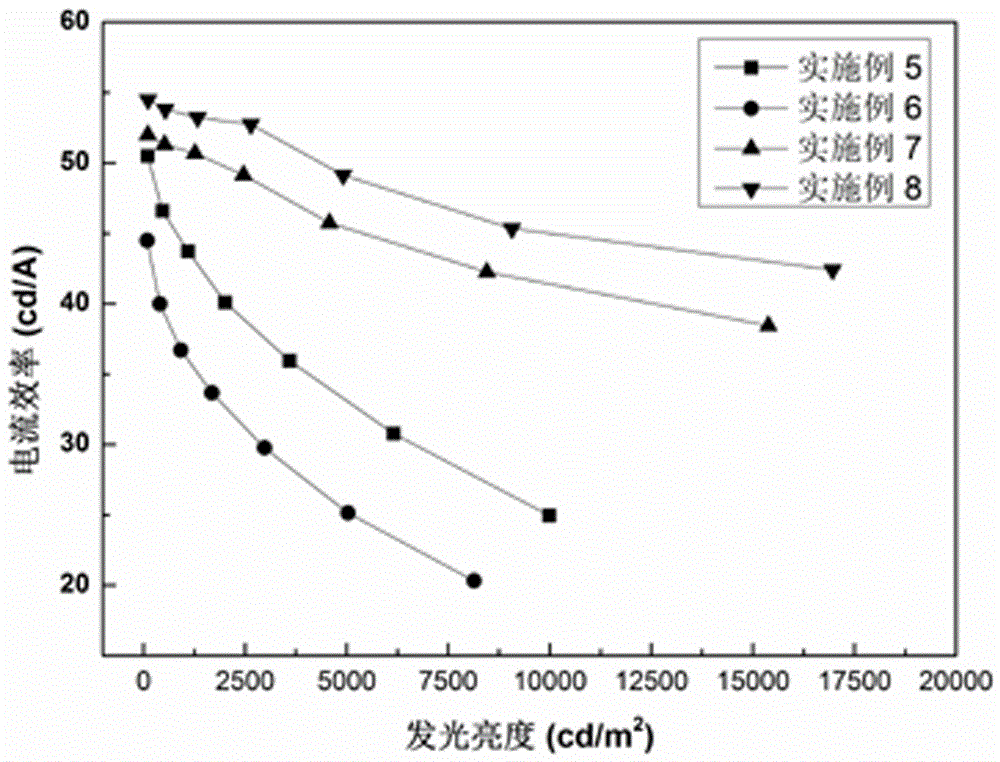
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com