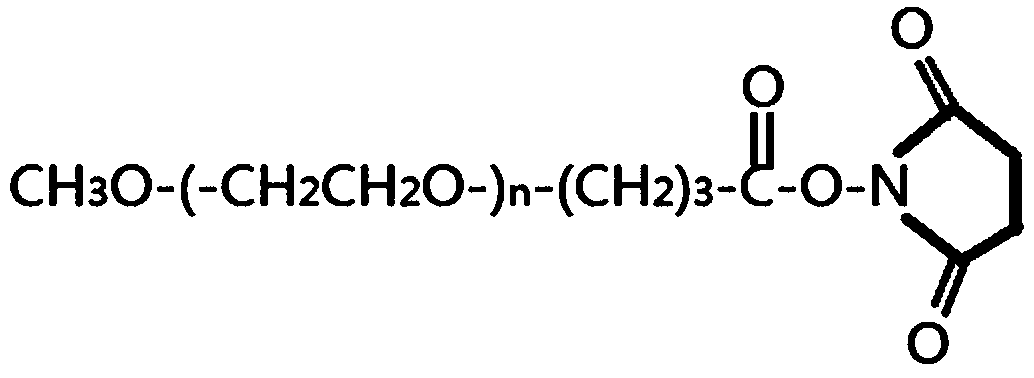Preparation method of single-modified polyethylene glycol recombinant human erythropoietin, its products and applications
A technology of polyethylene glycol and erythropoietin, which is applied to the preparation method of peptides, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of low single substitution rate, high multiple substitution rate, Purification is difficult and other problems, to achieve the effect of high recovery, simple process, and beneficial to purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] 2. Preparation of EPO stock solution
[0041] 2.1 Engineered cells
[0042] 2.1.1 Name and source
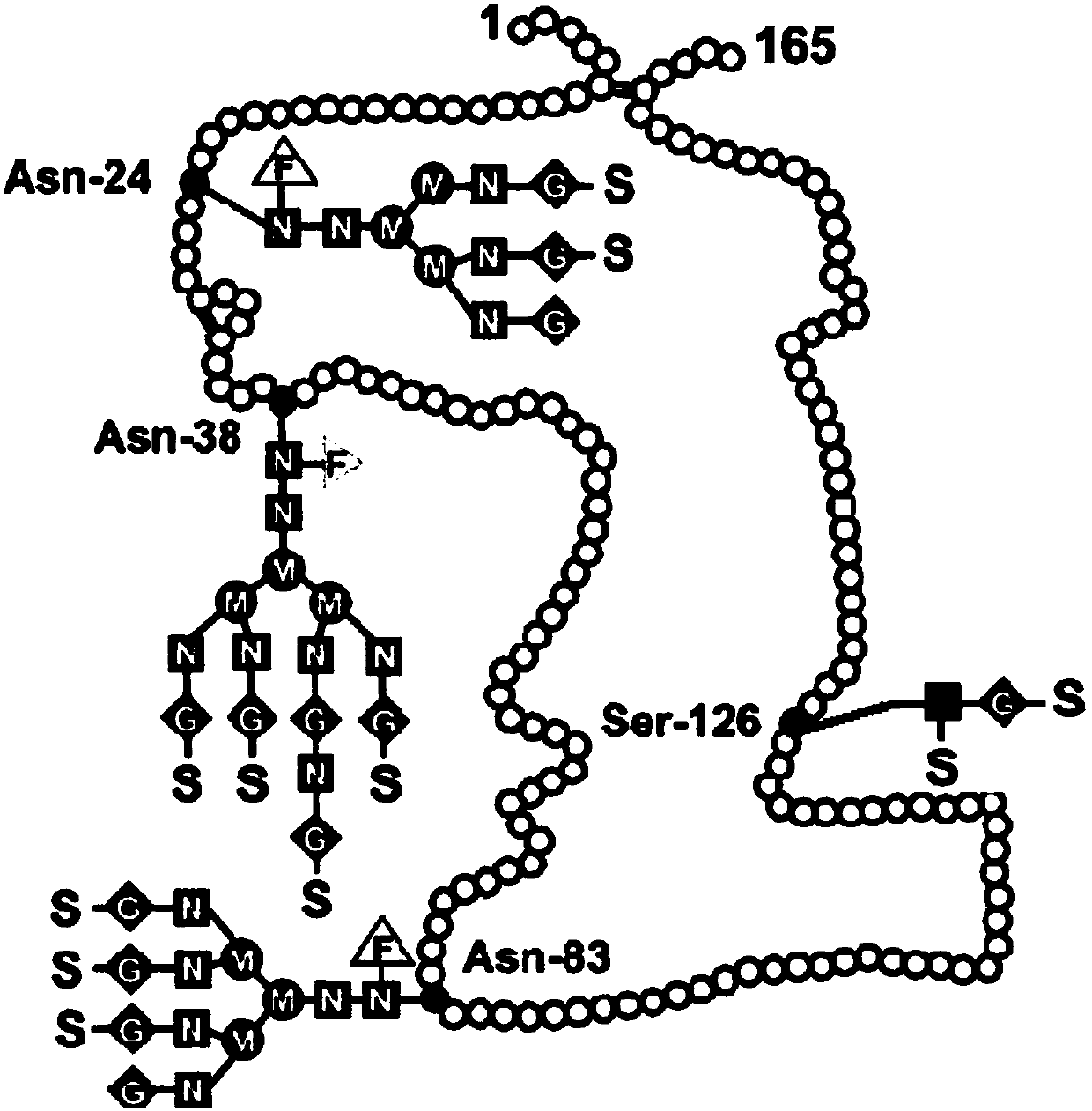
[0043] The polyethylene glycol recombinant human erythropoietin engineering cell line is a CHO-dhfr- (dihydrofolate reductase gene-deficient cell) cell line transfected with a recombinant plasmid carrying a human erythropoietin gene.
[0044] 2.1.2 Establishment, subculture and preservation of cell bank
[0045] Cells from the original cell bank are passaged, amplified and frozen in liquid nitrogen. As a master cell bank, the master cell bank should not exceed 2 cell passages at most; cells from the master cell bank are passaged, expanded and then frozen in liquid nitrogen. In nitrogen, as a working cell bank, the working cell bank must be limited to 1 cell passage. The cells are frozen in liquid nitrogen and can be used for production after passing the test.
[0046] 2.1.3 Verification of master cell bank and working cell bank cells
[0047] It should comply with th...
Embodiment 1
[0082] Example 1 Preparation of Monomodified Polyethylene Glycol Recombinant Human Erythropoin (PEG-EPO)—PEG Modification Reaction
[0083] Get the qualified EPO stock solution, adjust the buffer system to 150mM K 2 HPO 4 , pH7.5, EPO concentration is 2.84mg / ml.
[0084] According to the ratio of EPO:PEG molar ratio of 1:3 (mass ratio of 1:5), weigh mPEG-SBA (methoxypolyethylene glycol succinimide butyrate, straight chain, 30KDa, molecular structure see figure 2 ), added to the adjusted EPO stock solution, stirred slowly to dissolve, and stirred at a uniform speed (80-130rpm) with an electric stirrer at room temperature (20-25° C.), and reacted for 2 hours. After the reaction, dilute 5 times and adjust the pH to 4.0 with glacial acetic acid, and store in a refrigerator at 2-8°C for no longer than 3 days; if it exceeds 3 days, it needs to be stored in a refrigerator at -20°C.
Embodiment 2
[0085] Example 2 Identification of single-modified polyethylene glycol recombinant human erythropoietin—PEG modification reaction
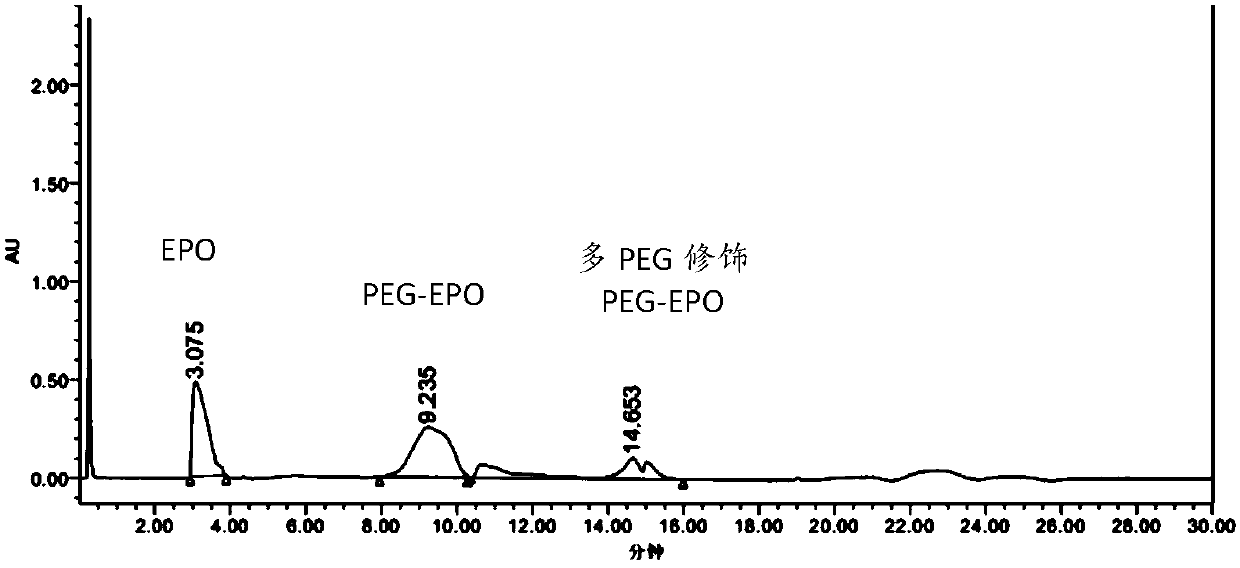
[0086] The PEG modification ratio of the single-modified polyethylene glycol recombinant human erythropoietin prepared in Example 1 was identified by reversed-phase-high performance liquid chromatography. Mobile phase A is trifluoroacetic acid aqueous solution (0.3% TFA); Mobile phase B is trifluoroacetic acid and acetonitrile aqueous solution (0.2% TFA, 84% acetonitrile); Flow rate is 1ml / min; Detection wavelength is 220nm; Column temperature is 60 ℃ ; The injection volume is 5-20μg. The chromatographic column adopts Agilent Poroshell 300SB C8 (5μm, 300A, 2.1x75mm).
[0087] Elution gradient:
[0088]
[0089]
[0090] The identification results of PEG modification ratio are shown in image 3 And table 1, target product single modification PEG-EPO (structural formula sees Figure 4 ) was 42.91%.
[0091] Table 1 Chromatographic peak re...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com