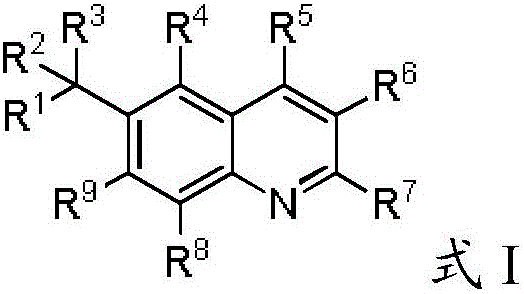
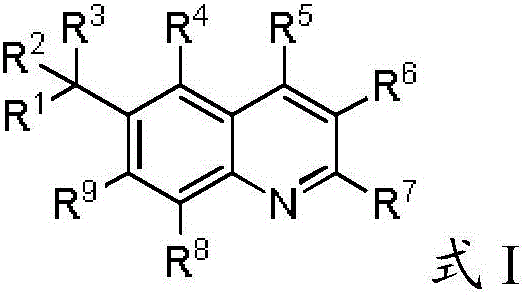
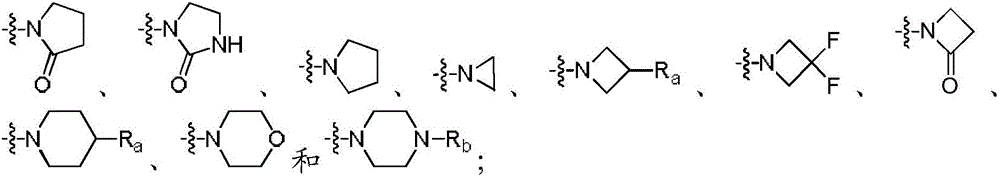Phenyl Linked Quinolinyl Modulators Of Ror-Gamma-T
A phenyl and methyl technology, applied in anti-inflammatory agents, non-central analgesics, medical preparations containing active ingredients, etc., can solve the problems of EAE sensitivity reduction and Th17 cell population reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1a
[0545] Example 1a: 1-(4-((4-Chloro-2-methoxy-3-(3-(trifluoromethyl)phenyl)quinolin-6-yl)(hydroxyl)(1- Methyl-1H-imidazol-5-yl)methyl)piperidin-1-yl)ethanone
[0546]
[0547]n-Butyllithium (1.6M in hexane, 0.585 mL, 0.937 mmol) was added to 6-bromo-4-chloro-2-methoxy-3-(3-(tri Fluoromethyl)phenyl)quinoline (409.8 mg, 0.984 mmol, Intermediate 5: step e) in THF (3 mL). After 1 min, 1-(4-(1-methyl-1H-imidazol-5-carbonyl)piperidin-1-yl)ethanone (231.4 mg, 0.984 mmol, Intermediate 1: step c) was added via cannula solution in THF (5 mL). The mixture was stirred at -78°C for 10 minutes, then transferred to an ice bath and stirred for an additional 45 minutes. Add saturated NH 4 The reaction mixture was quenched with aqueous Cl, diluted with water and extracted with EtOAc (3x). dry (Na 2 SO 4 ) organic phase, filtered and concentrated to dryness. The residue was purified by flash column chromatography (silica gel, 0-8% MeOH-DCM) to afford the title compound as a white so...
Embodiment 1b
Embodiment 2a
[0549] Example 2a: 6-((1-acetylpiperidin-4-yl)(hydroxyl)(1-methyl-1H-imidazol-5-yl)methyl)-2-methanol Oxy-3-(3-(trifluoromethyl)phenyl)quinoline-4-carbonitrile
[0550]
[0551] A round bottom flask was charged with 1-(4-((4-chloro-2-methoxy-3-(3-(trifluoromethyl)phenyl)quinolin-6-yl)(hydroxy)(1 -Methyl-1H-imidazol-5-yl)methyl)piperidin-1-yl)ethanone (158mg, 0.276mmol, Example 1a), Zn(CN) 2 (58.3mg, 0.496mmol), Pd 2 (dba) 3 (37.9mg, 0.041mmol), nano zinc powder (5.4mg, 0.083mmol) and dicyclohexyl (2',4',6'-triisopropyl-[1,1'-diphenyl]-2- yl)phosphine (X-Phos, 27.1 mg, 0.055 mmol). The flask was evacuated and refilled with argon (three cycles). Dimethylacetamide (1.4 mL, under argon for 30 minutes) was then added, and the mixture was heated at 120° C. for 4 hours. The mixture was cooled to room temperature, filtered Washed with EtOAc. 2MNH 4 The filtrate was washed with aqueous OH, water and saturated aqueous NaCl. dry (Na 2 SO 4 ) organic phase, filtered and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com