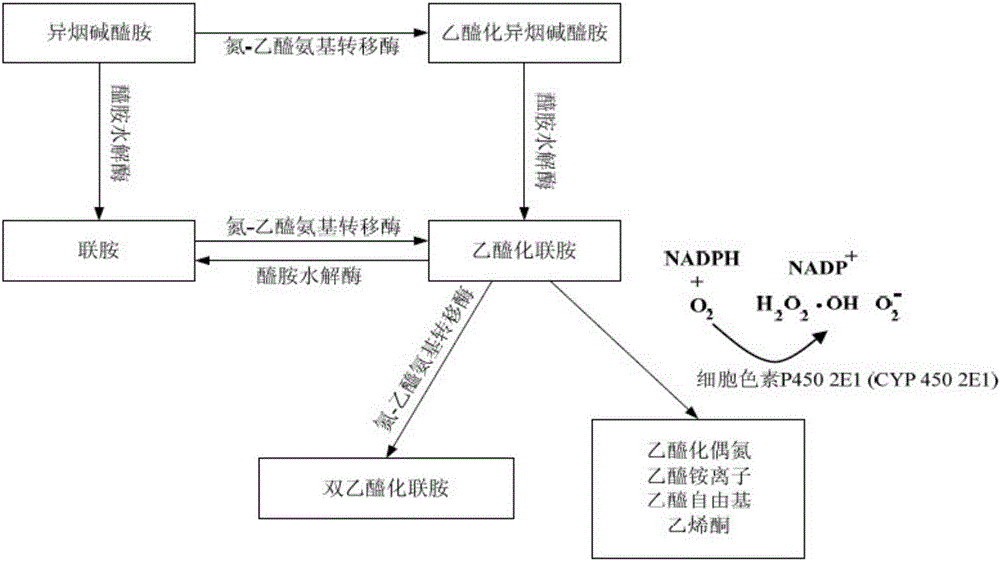
Antitubercular compound drug with no/low side effect
A technology for tuberculosis and side effects, applied in the field of compound anti-tuberculosis drugs, can solve problems such as non-good design, achieve obvious inhibitory activity and reduce liver toxicity and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
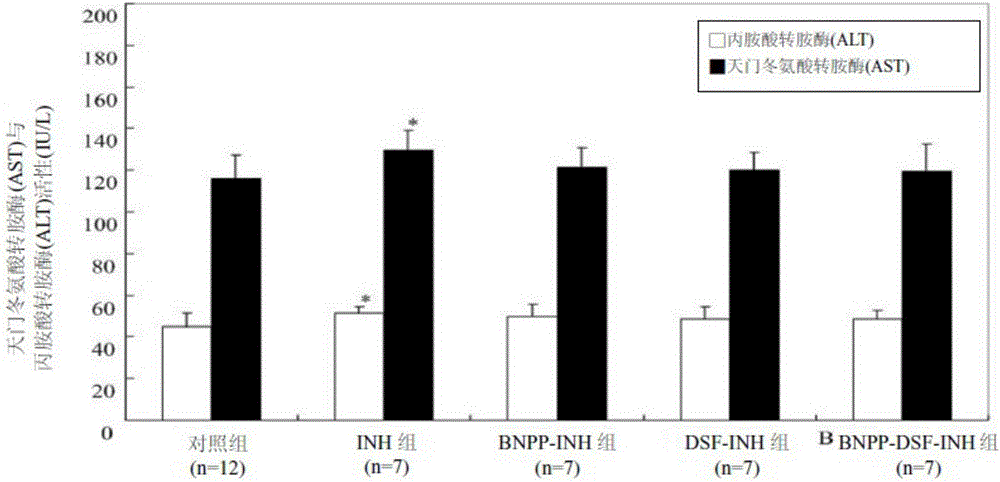
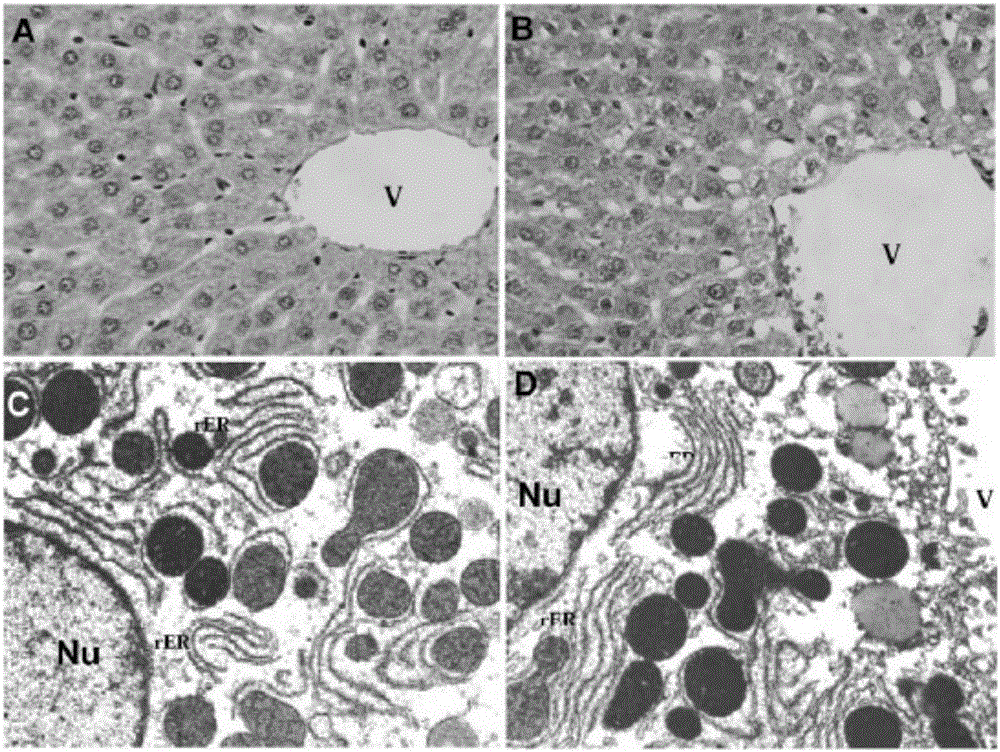
[0041] Example 1 Animal experiment of isonicotinamide (INH) combined with CYP2E1 inhibitor disulfiram (DSF) and / or nitrophenol phosphate diester (BNPP)
[0042] 1. Materials and methods
[0043] 1. Test material
[0044] All organic solvents were HPLC grade purchased from Tedia Co., Ltd. (Fairfield, OH, USA), INH, BNPP, DSF and corn oil were purchased from Sigma Chemical Company (St.Louis, MO USA), 8-iso-PGF 2α and radiographically calibrated 8-iso-PGF 2α -d 4 It was obtained from Cayman Chemical Company (Ann Arbor, MI, USA), and the galactose injection solution was prepared by Nanguang Chemical Pharmaceutical Co., Ltd. by dissolving 400 grams of galactose (Sigma) in 1 liter of an appropriate buffer solution system and isotonic saline. Class distilled water for injection use.
[0045] 2. Test animals
[0046] Male SD (Sprague-Dawley) rats with a body weight of 320-350 grams were purchased from the National Laboratory Animal Center (Taiwan). The animal experiments were car...
Embodiment 2
[0088] Example 2 Screening of Cytochrome P450 2E1 (CYP2E1) Inhibitors-
[0089] cDNA synthesis of microsomal cytochrome P450 2E1.
[0090] 1. Materials and methods
[0091] 1. Test material
[0092] This example uses the screening kit of cytochrome P450 2E1 (CYP2E1) inhibitors (CYP2E1 High Throughput Inhibitor Screening Kit, BD Bioscience, USA) to conduct cytochrome P450 2E1 (CYP2E1) Screening of inhibitors; the principle of action of the screening kit of CYP2E1 inhibitors is as follows: adding cytochrome P450 2E1 (CYP2E1) and its fluorescent substrate MFC (7-Methoxy-4-trifluoromethyl coumarin) to the environment After the test sample has acted, detect the generation of CYP2E1 metabolite standard HFC (7-Hydroxy-4-trifluoromethyl coumarin), and calculate the CYP 2E1 inhibition rate of the test sample based on the HFC generation of the control group (control) .
[0093] Each test sample was dissolved in acetonitrile (acentoitrile), and the inhibition rate of CYP2E1 was teste...
Embodiment 3
[0140] Example 3 Screening of Cytochrome P450 2E1 (CYP2E1) Inhibitors - Human Liver Microsomal Cytochrome P450 2E1
[0141] 1. Materials and methods
[0142] 1. Test material
[0143]In this example, microsomes prepared from human liver were used to screen cytochrome P450 2E1 (CYP2E1) inhibitors against cytochrome P450 2E1 (CYP2E1), 39 kinds of traditional Chinese medicines and 10 kinds of excipients, so as to screen out the inhibitors of cytochrome P450 2E1 (CYP2E1) An effective inhibitor of cytochrome P450 2E1 (CYP2E1) in the liver; the screening principle of the CYP2E1 inhibitor is as follows: the cytochrome P450 2E1 (CYP2E1) in microsomes prepared from human liver reacts with its substrate Chlorzoxazone, and after adding the test sample, Then detect the production of CYP2E1 metabolite standard 6-OH-CZX (6-Hydroxy-Chlorzoxazone), and calculate the CYP 2E1 inhibition rate of the test sample based on the production of 6-OH-CZX in the control group (control) .
[0144] Each...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com