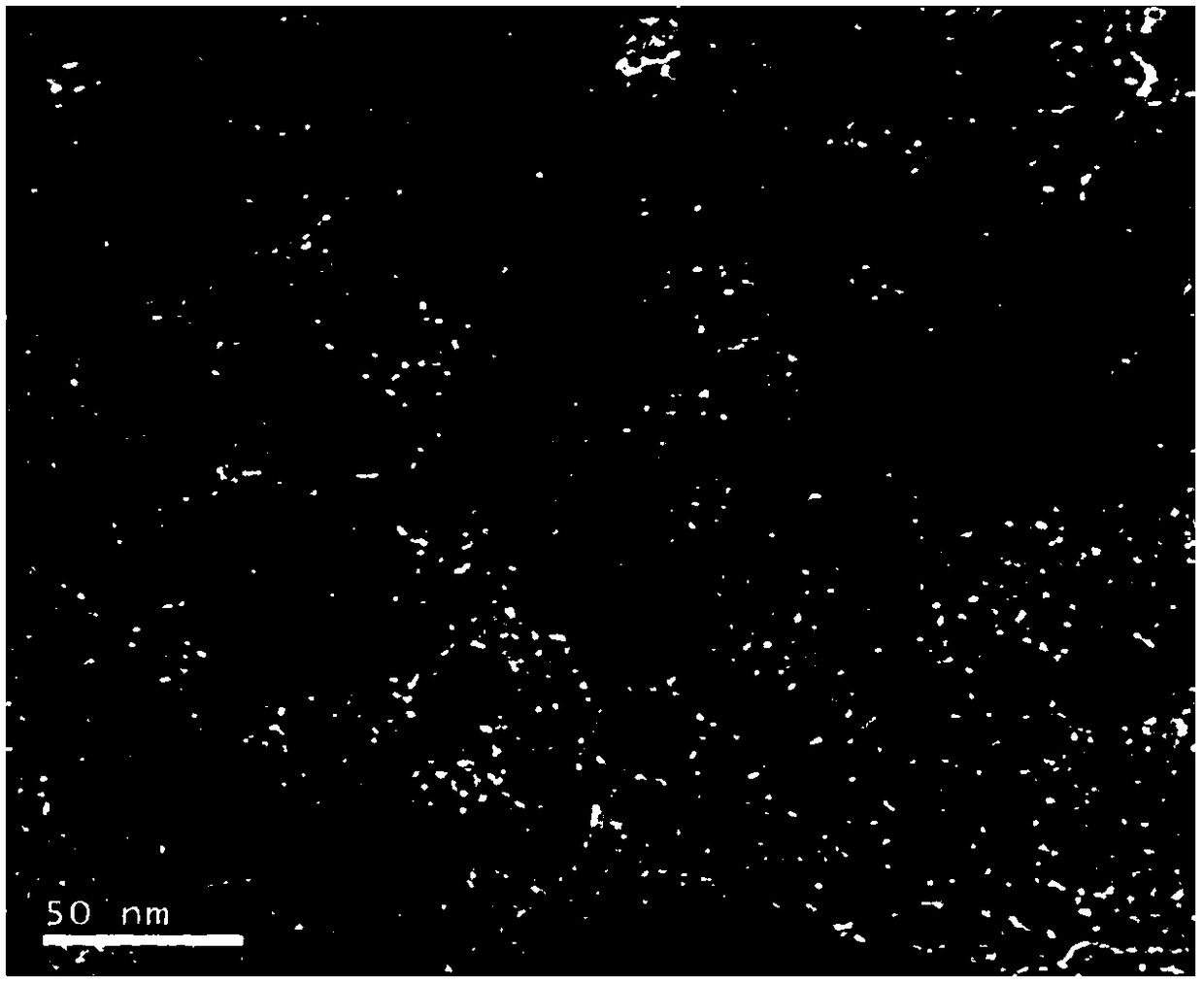
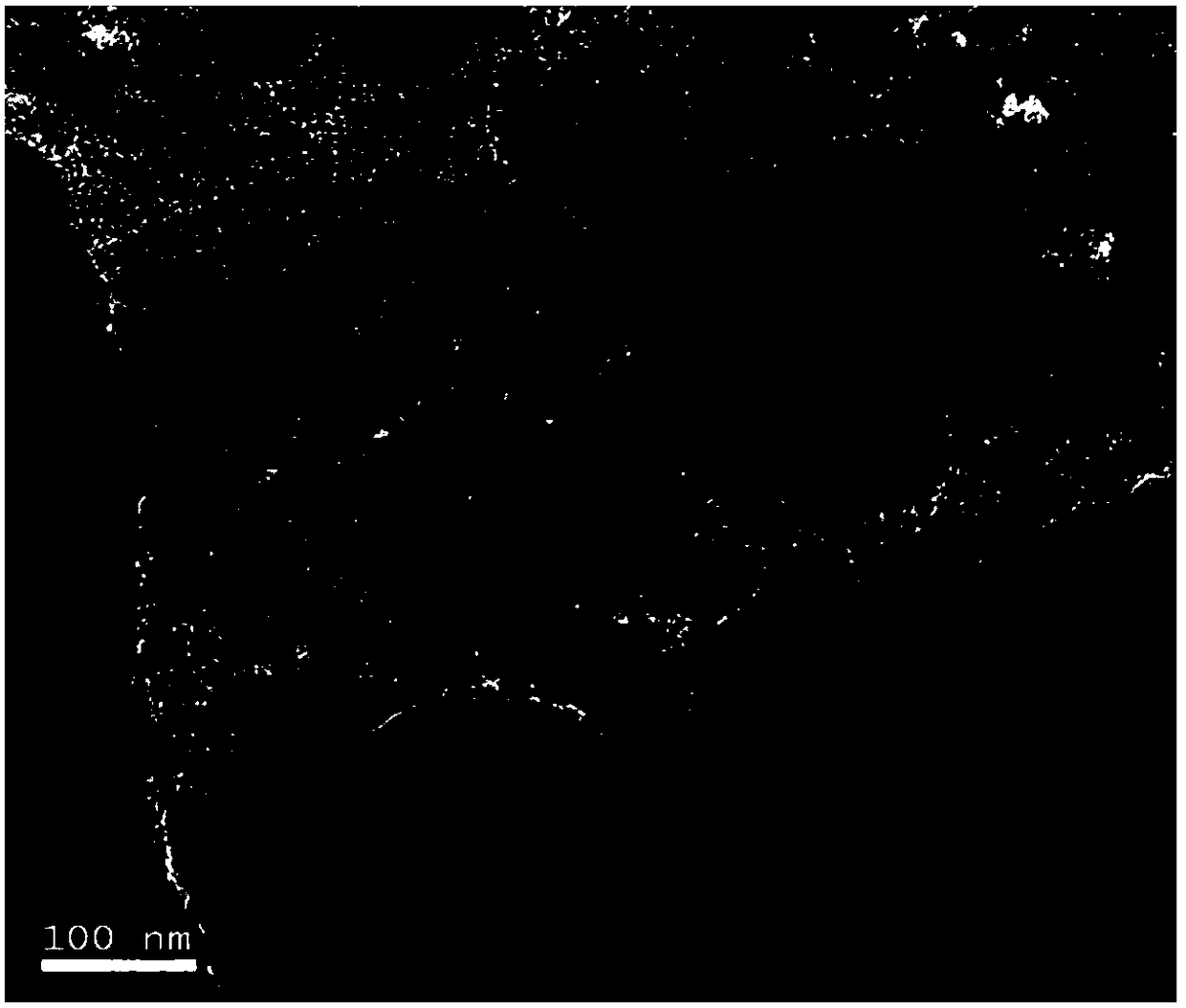
A kind of supported spherical nanoparticle palladium catalyst and its preparation method and application
A nanoparticle and palladium catalyst technology, applied in the field of supported spherical nanoparticle palladium catalyst and its preparation, can solve the problems that hinder the breakthrough of industrial production technology, the requirements of improving precision and difficulty, and the reaction end point is not obvious, and reach the reaction end point Ease of use, easy operation, and many times of application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~ Embodiment 15
[0048] The content, proportion and preparation conditions of the catalyst active components are provided (as shown in Table 1).
[0049] Catalyst concrete preparation process is as follows (taking embodiment 1 as example):
[0050] 1) Dry and dehydrate the activated carbon made from coconut shell in vacuum at 120°C for 3 hours;
[0051] 2) Dissolve palladium chloride in concentrated ammonia water (25-28wt%) in an amount that just dissolves (1.0 times the theoretical amount), stir at room temperature until completely dissolved, and then dilute with water to prepare a concentration of 0.05g / ml of palladium solution.
[0052] 3) Take 25ml of ethylene glycol solution with a mass content of 80% in a 100ml hydrothermal synthesis kettle, and control the temperature at -8.0°C. Finally, pour 10 g of activated carbon that has been vacuum-dried and dehydrated into the synthesis kettle, and stir until the temperature of the slurry reaches -8.0°C; drop in 10ml of the palladium solution ...
Embodiment 16~32
[0061] 50g m-dinitrobenzene, 100ml methyl alcohol and 0.5g embodiment 1~15 or the loaded catalyst prepared by comparative example 1~2 are added in the 500mL autoclave, close the reactor, replace the air in the reactor with nitrogen, and then The nitrogen is replaced with hydrogen, the stirring is started, the stirring speed is 1000r / min, the reaction temperature is maintained at 25-70°C, and the hydrogen pressure is 1.0-1.5MPa to carry out the reaction. When the hydrogen gas no longer drops within 5 minutes, it is considered that the reaction is terminated, the reaction is stopped, and the catalyst is filtered. The filtrate is the product after phase separation, water separation and dehydration by vacuum distillation, and it is quantitatively analyzed by chromatography (mole percentage).
Embodiment 33
[0063] The catalyst prepared in Example 10 is selected, the carrier particle size is 0.5-1.0 mm, and the fixed bed is 8 cm. The gas space velocity of the sum of hydrogen and m-dinitrobenzene is 2.0*10 4 h -1 , the molar ratio of hydrogen to m-dinitrobenzene is 40:1; the temperature is 70°C, and the hydrogen pressure is 2.0MPa. The product was analyzed by chromatography, and the conversion and selectivity were both 100%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com