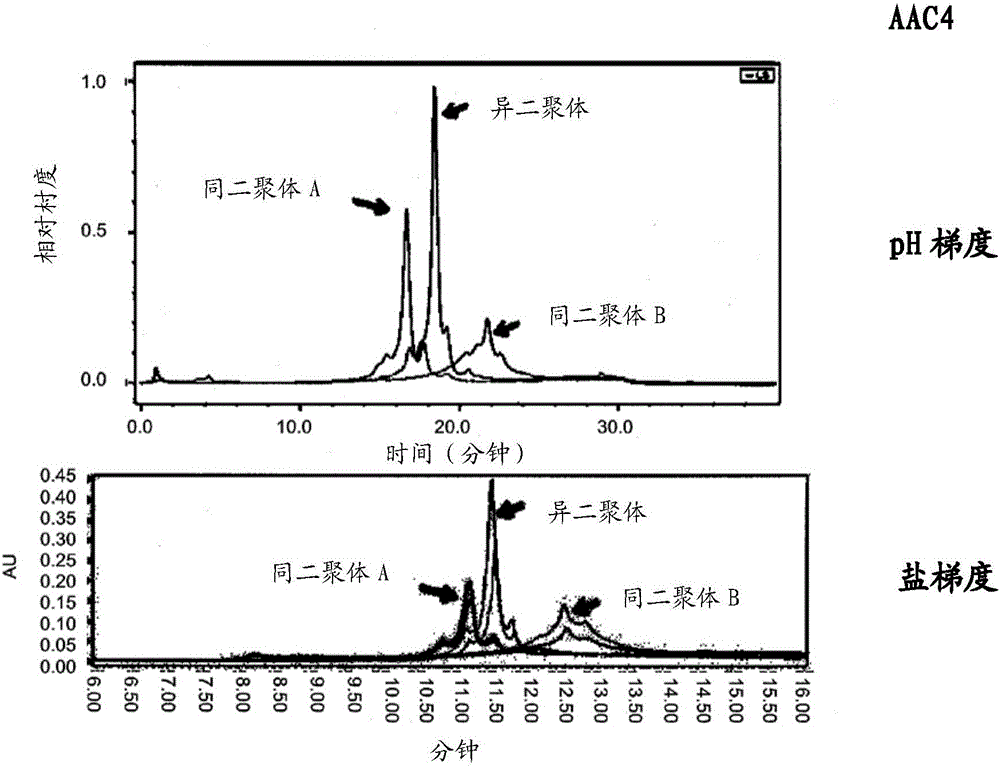
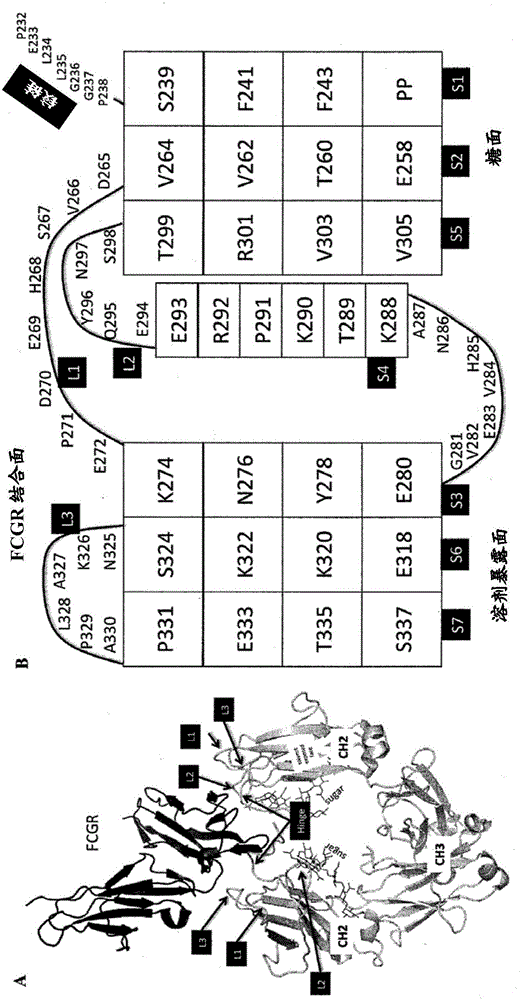
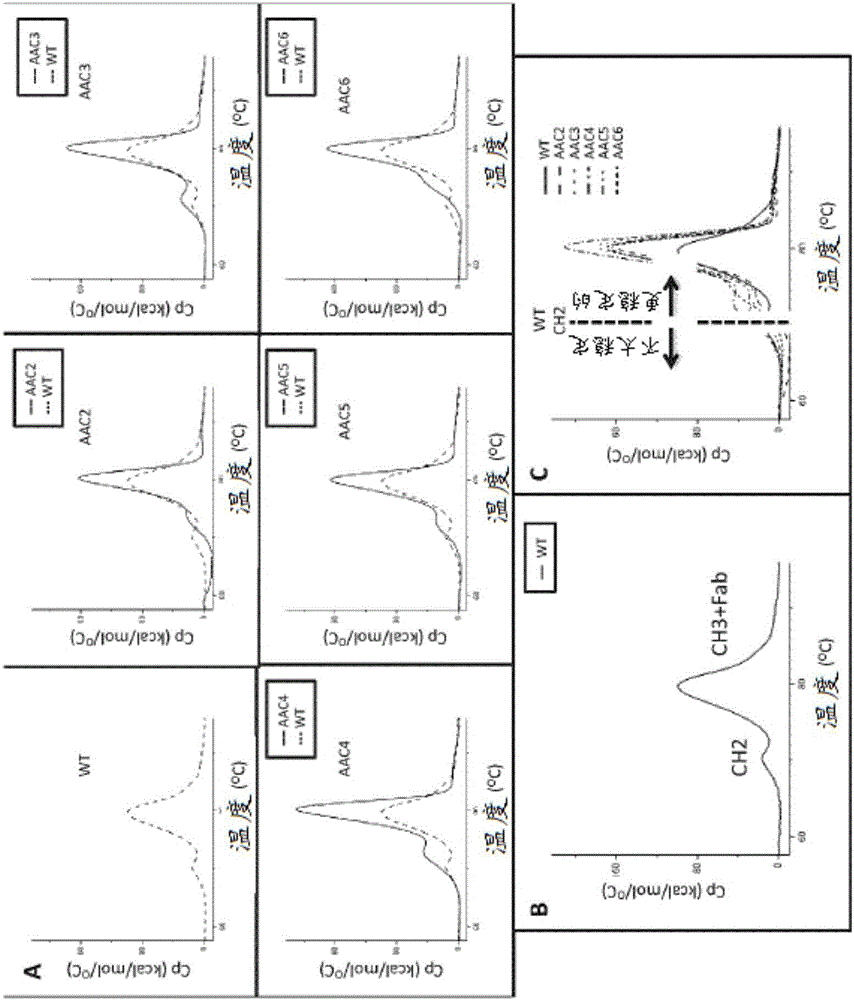
Heteromultimers with reduced or silenced effector function
An effector function and multimer technology, applied in the field of polypeptides containing heterodimeric Fc regions, can solve problems such as adverse side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0263] Example 1: Preparation and expression of antibody constructs (heteromultimers)
[0264] The following antibody constructs were prepared. All antibody constructs were based on the sequence of the wild-type anti-Her2 antibody trastuzumab (see Figure 5 , SEQ ID NO:2 is the amino acid sequence of the wild-type trastuzumab heavy chain, and SEQ ID NO:3 is the amino acid sequence of the wild-type trastuzumab light chain), introducing the following modifications added in the heavy chain CH3 domain , to promote the formation of a heterodimeric Fc domain with increased stability compared to a CH3 domain that does not contain amino acid mutations.
[0265] Chain A: T350V / L351Y / S400E / F405A / Y407V, and
[0266] Chain B: T350V / T366L / N390R / K392M / T394W
[0267] This construct with the above modifications was designated v791. All sequences described herein are numbered using the EU numbering system.
[0268] Additional variants were constructed based on v791 with amino acid modific...
Embodiment 2
[0280] Example 2: Trastuzumab-based asymmetric antibodies do not bind FcγRs
[0281] The ability of asymmetric antibody constructs to bind to FcyRIIaH, FcyRIIaR, FcyRIIb FcyRIIIaF, FcyRIIIaV and FcyRIa was assessed by surface plasmon resonance (SPR).
[0282] Affinity of FcyRs to antibody Fc was measured by SPR using a ProteOn XPR36 using 10 mM HEPES, pH 7.4, 150 mM NaCl, 3.4 mM EDTA, and 0.05% Tween 20 at 25°C. Recombinant HER-2 was captured on an activated GLM sensor chip as follows: 4.0 μg / mL recombinant HER-2 in 10 mM NaOAc (pH 4.5) was injected at 25 μL / min until approximately 3000 resonance units were immobilized. , RU), to quench the remaining active groups. After injection with buffer to establish a stable baseline, 40 μg / mL of purified HER-2 / neu-based antibody was captured indirectly with an injection at 25 μL / min for 240 s (resulting in approximately 500 RU). FcyRs were injected at 60 μL / min for 120 seconds with a 180 second dissociation phase to obtain a set of bi...
Embodiment 3
[0294] Example 3: Trastuzumab-based asymmetric antibodies do not bind C1q
[0295] Asymmetric antibody constructs were tested for their ability to bind CIq as follows. Human C1q was purchased from GenWay Biotech (San Diego, CA). SPR chip immobilization of antibodies was performed as described in Example 2. Also as described in Example 2, 30 nM C1q was injected over the mAb variants captured on the surface of the HER2 SPR using standard protocols. The results are shown in Table C below.
[0296] Table C: Results of the C1q binding assay
[0297] Variants
C1q 1
WT
yes
Control / 1051
NB
AAC1
part
AAC2
NB
AAC3
NB
AAC4
NB
AAC5
NB
AAC6
NB
AAC7
NB
AAC8
NB
[0298] 1. C1q is a hexamer of heterotrimers with a possible stoichiometric ratio of mAb:C1q of 6:1. Binding kinetics are very complex and the appropriate Kd may not be determined. Receptors were tes...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com