Blood preservation solution
A technology of blood preservation solution and preservation solution, which is applied in the field of cell therapy and can solve the problems of verification of the subsequent proliferation ability of lymphocytes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
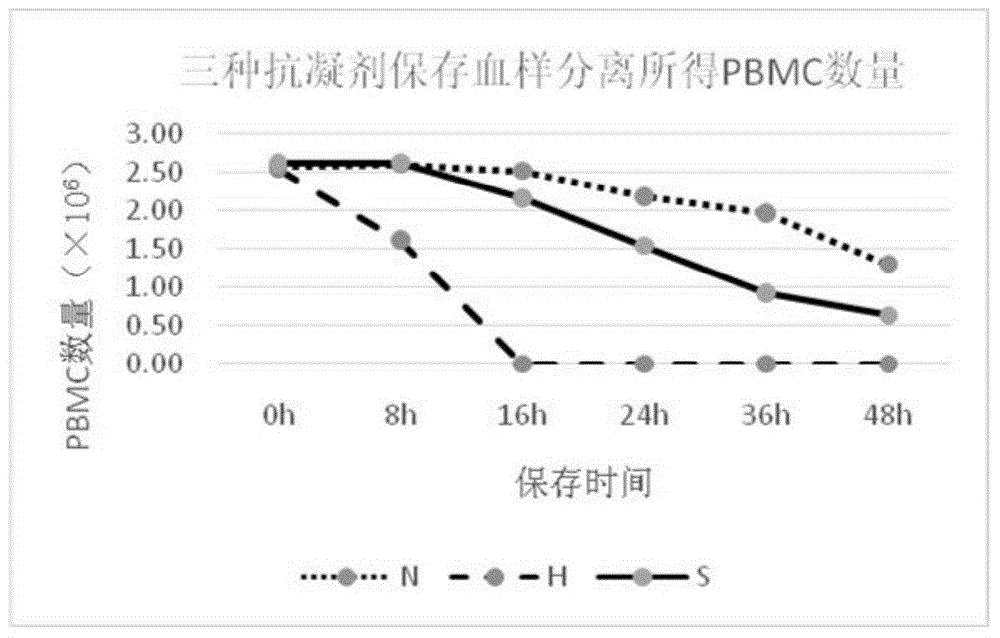
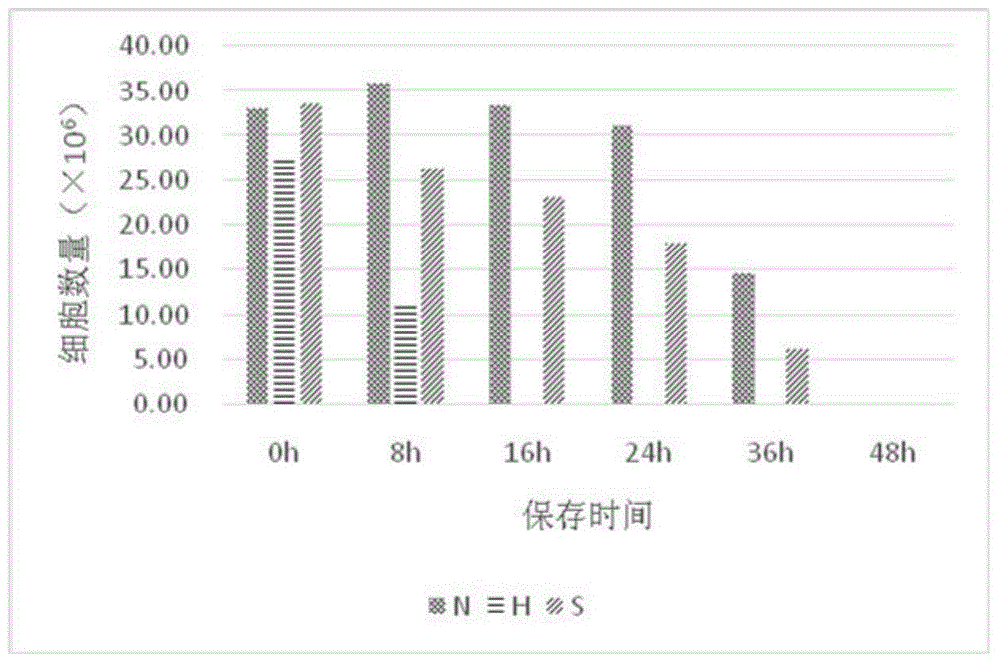
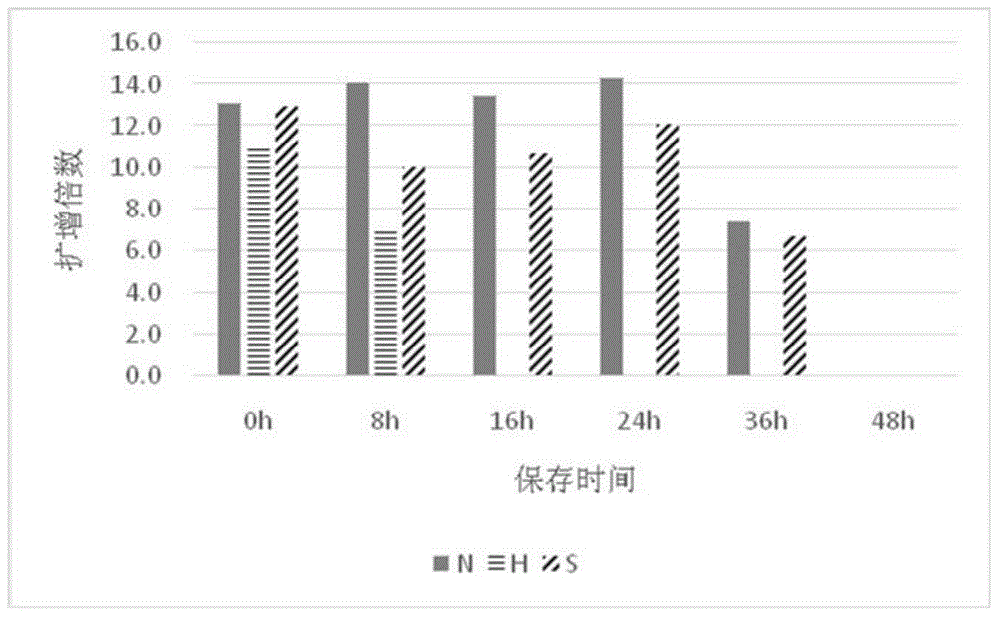
[0033] Embodiment 1, three kinds of anticoagulants / preservation solution performance comparison
[0034] 1. Preservation solution preparation
[0035] The heparin anticoagulant (herein referred to as H) is a lithium heparin vacuum anticoagulant tube (Greiner, 9ml).
[0036] The formula of the anticoagulant disclosed in CN 1411818A (hereinafter referred to as S) is as follows in Table 1:
[0037] Formulation S in Table 1: per 100ml
[0038]
[0039] The specific preparation method is as follows (20ml): first dissolve 664mg of trisodium citrate, 5mg of citric acid and 550mg of glucose for injection in 12ml. Then dilute to 20ml with water for injection. Adjust the pH to 6.8-7.3 using NaOH and HCl. Filter with a 0.22um needle filter, then aliquot 5ml / tube, and store at 4°C.
[0040] Preservation solution of the present invention (herein referred to as N) formula is as follows table 2:
[0041] Formulation N in Table 2: per 100ml
[0042]
[0043]The specific preparati...
Embodiment 2
[0068] Embodiment 2, preservation solution formulation optimization
[0069] On the basis of Example 1, the inventors studied the amount of SOD in formula N, so as to optimize the protective performance of the preservation solution on lymphocytes.
[0070] Peripheral blood (54ml / person) was collected from three healthy donors, and the blood samples were stored at 2-8°C in preservation solutions containing three concentrations of SOD (70U / 100ml, 280U / 100ml, 560U / 100ml) at a ratio of 1 : 10 (preservation solution: peripheral blood). 3ml of peripheral blood was taken out at five time points of 0h, 12h, 24h, 36h, and 48h, and peripheral blood mononuclear cells (PBMCs) were separated by Ficoll-Hypaque (GE) density gradient centrifugation, and samples were taken for detection. Cell culture medium (IMSF100, Immunotech UK.Ltd) was resuspended and inoculated into culture flasks coated with activated antibodies (Ebioscience) for expansion culture. The cell culture medium was replenish...
Embodiment 3
[0091] Embodiment 3, optimization of preservation solution usage ratio
[0092] On the basis of the foregoing examples, the inventors studied the proportion of formula N used so as to optimize the protective performance of the preservation solution on lymphocytes.
[0093]Collect peripheral blood (54ml / person) from three healthy donors, mix with the preservation solution of the present invention in three proportions respectively (preservation solution: blood sample=1:20, 1:10, 1:5), and store the blood sample at 2-8 ℃. 3ml of peripheral blood was taken out at five time points of 0h, 12h, 24h, 36h, and 48h, and peripheral blood mononuclear cells (PBMCs) were separated by Ficoll-Hypaque (GE) density gradient centrifugation, and samples were taken for detection. Cell culture medium (IMSF100, Immunotech UK.Ltd) was resuspended and inoculated into culture flasks coated with activated antibodies (Ebioscience) for expansion culture. The cell culture medium was replenished once at 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com