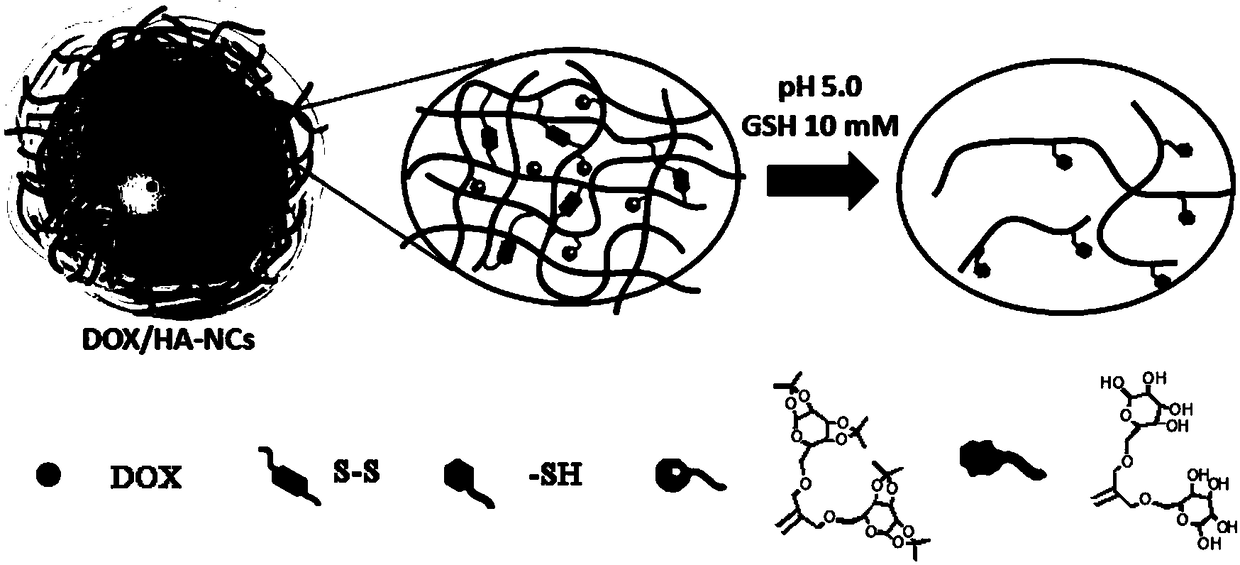
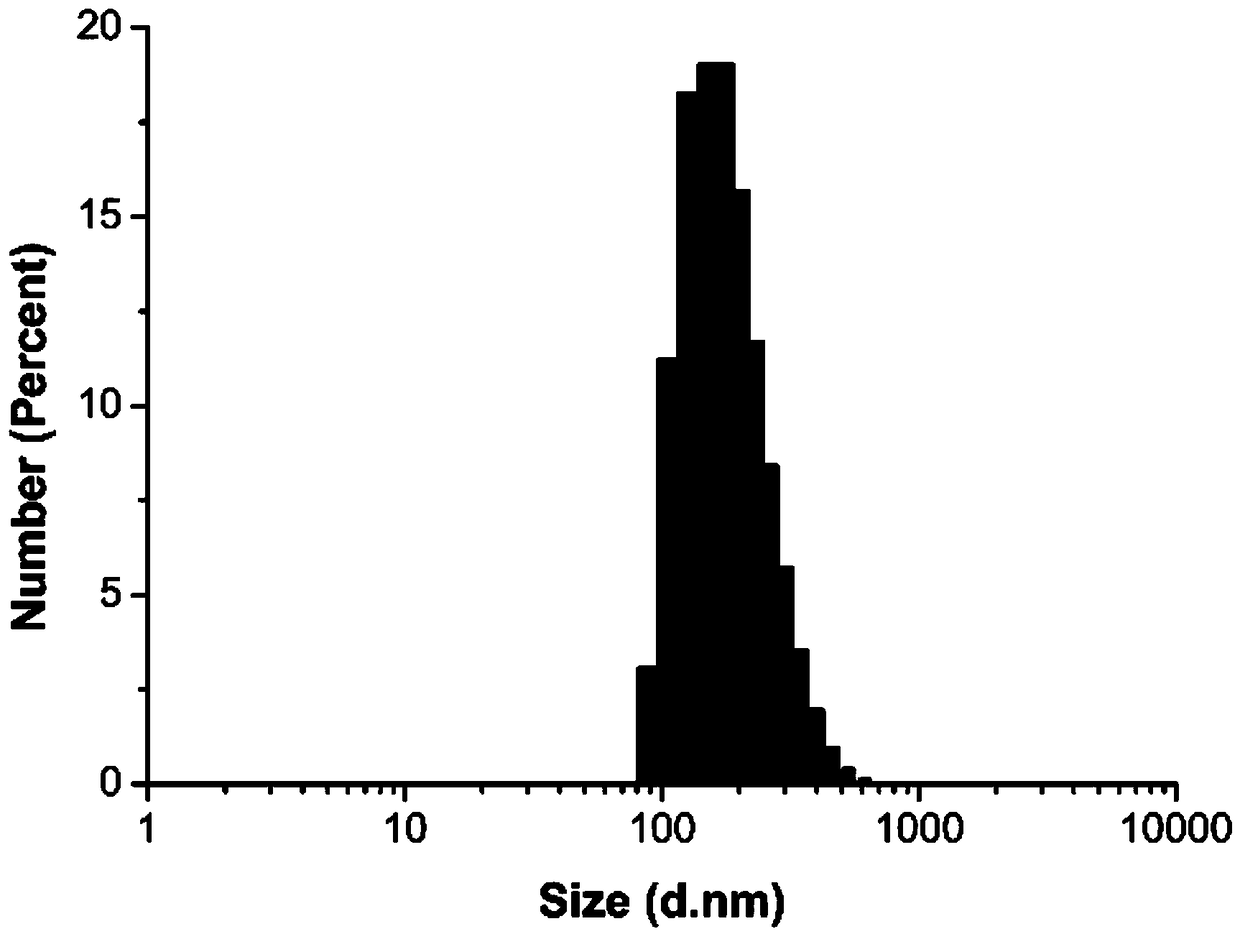
A dual cell microenvironment sensitive anti-tumor drug-loaded nanocapsule and its preparation method
A cell microenvironment, drug-loaded nanotechnology, applied in the field of biomedical materials, can solve the problems of poor anti-tumor effect and safety, complicated operation, slow drug release rate from drug-loaded nanoparticles, etc. Effective, good biosafety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Preparation of mercaptolated hyaluronic acid
[0043] Dissolve hyaluronic acid (HA) in a phosphate buffer solution with a concentration of 0.01mol / L and pH=7.4, then add 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide and 1 -Hydroxybenzotriazole, activate for 2 hours, then add cystamine dihydrochloride dropwise, stir for 12 hours, dialyze the resultant in deionized water with a 3.5kD dialysis bag for 72 hours, remove small molecular substances such as condensing agents, and finally Dithiothreitol was added to break the formed disulfide bond, purified by deionized water dialysis again, and freeze-dried to obtain thiolated hyaluronic acid; among them, hyaluronic acid, 1-(3-dimethylaminopropyl) The molar ratios of -3-ethylcarbodiimide, 1-hydroxybenzotriazole, cystamine dihydrochloride and dithiothreitol are 1:3:3:3:5;
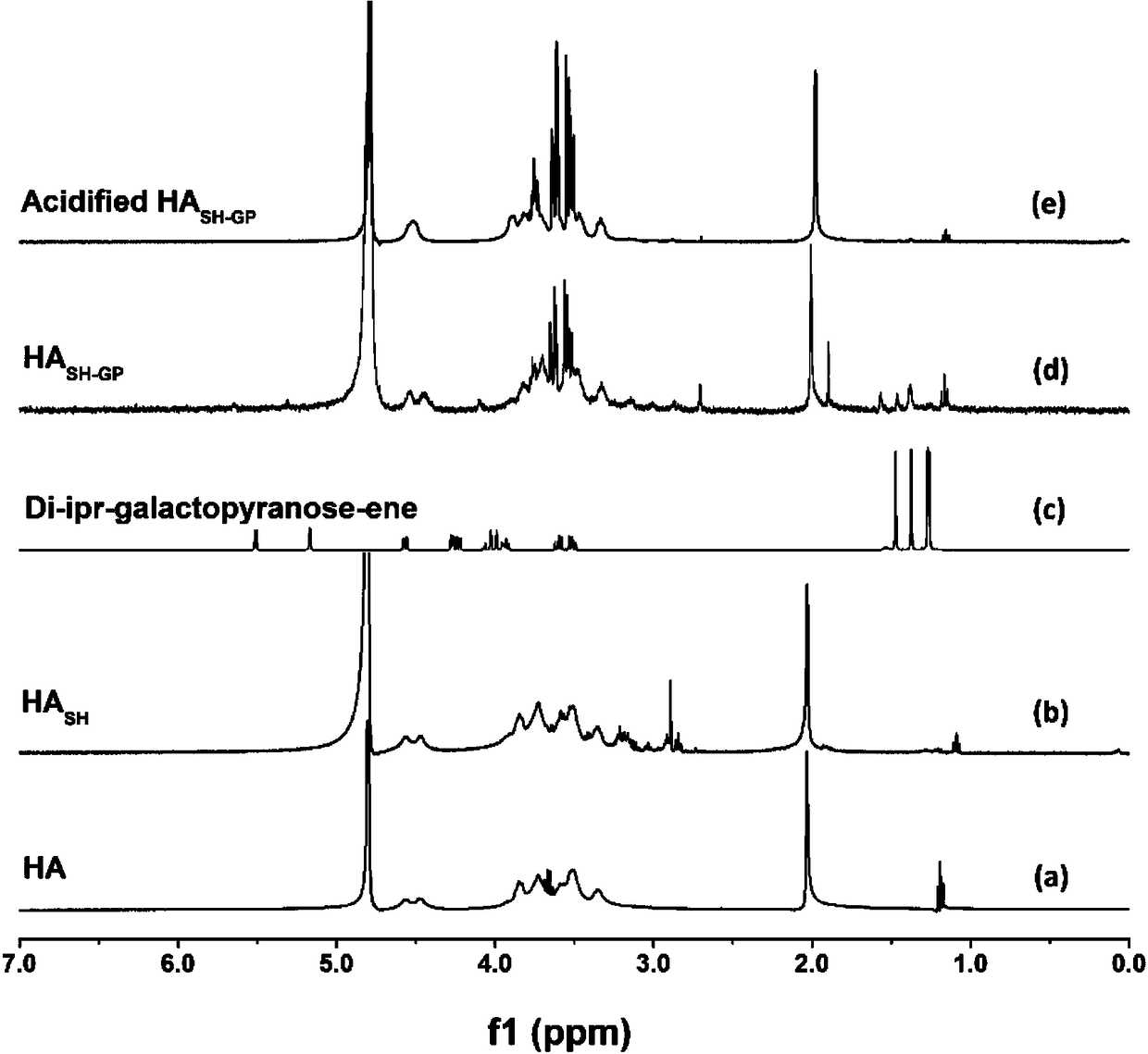
[0044] Separately dissolve hyaluronic acid and the resulting thiolated hyaluronic acid in D 2 O, do 400 MHz 1 H-NMR scan, see the results figu...
Embodiment 2
[0045] Example 2 Preparation of vinyl-functionalized second-generation isopropylidene-protected galactopyranose
[0046] Dissolve 3-chloro-2-chloromethylpropene and NaH in anhydrous tetrahydrofuran, then add isopropylidene-protected galactopyranose, and add a small amount of KI, 18-crown-6 (18-Crown- 6) and 15-crown-5 (15-Crown-5) as a catalyst, heated to reflux at 65-70°C for 24 hours, added water to terminate the reaction, then extracted the product with ethyl acetate, and removed the organic solvent by rotary evaporation, and then used ethyl acetate Esters: n-hexane = 1:3 (volume ratio) as the eluent, purified with silica gel chromatography, and removed the organic solvent to obtain vinyl-functionalized second-generation isopropylidene protected galactopyranose; where 3-chloro- The molar ratio of 2-chloromethylpropene, NaH, isopropylidene-protected galactopyranose and catalyst is 3:3.13:1:0.0075; wherein the catalyst is KI, 18-crown ether-6 and 15-crown ether- 5 Mix the re...
Embodiment 3
[0048] Example 3 Grafting of second-generation isopropylidene protected galactopyranose on mercaptolated hyaluronic acid
[0049] The mercaptolated hyaluronic acid and vinyl-functionalized second-generation isopropylidene protected galactopyranose were dissolved in a mixture of dimethylformamide and phosphate buffer at a volume ratio of 1:3, and then Add the photoinitiator 2,2-dimethoxy-2-phenylacetophenone (DAPA), and irradiate it with ultraviolet light with a wavelength of 365nm and a power of 6W for 2h. After the reaction is completed, dialyze with deionized water to remove The unreacted small molecular substances are finally freeze-dried to obtain products; among them, mercaptolated hyaluronic acid, vinyl-functionalized second-generation isopropylidene protected galactopyranose and 2,2-dimethoxy-2-phenyl The molar ratio of acetophenone is 1:0.5:0.05.
[0050] make the resulting product 1 H-NMR scanning, nuclear magnetic spectrum such as figure 2 As shown in d, after th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com