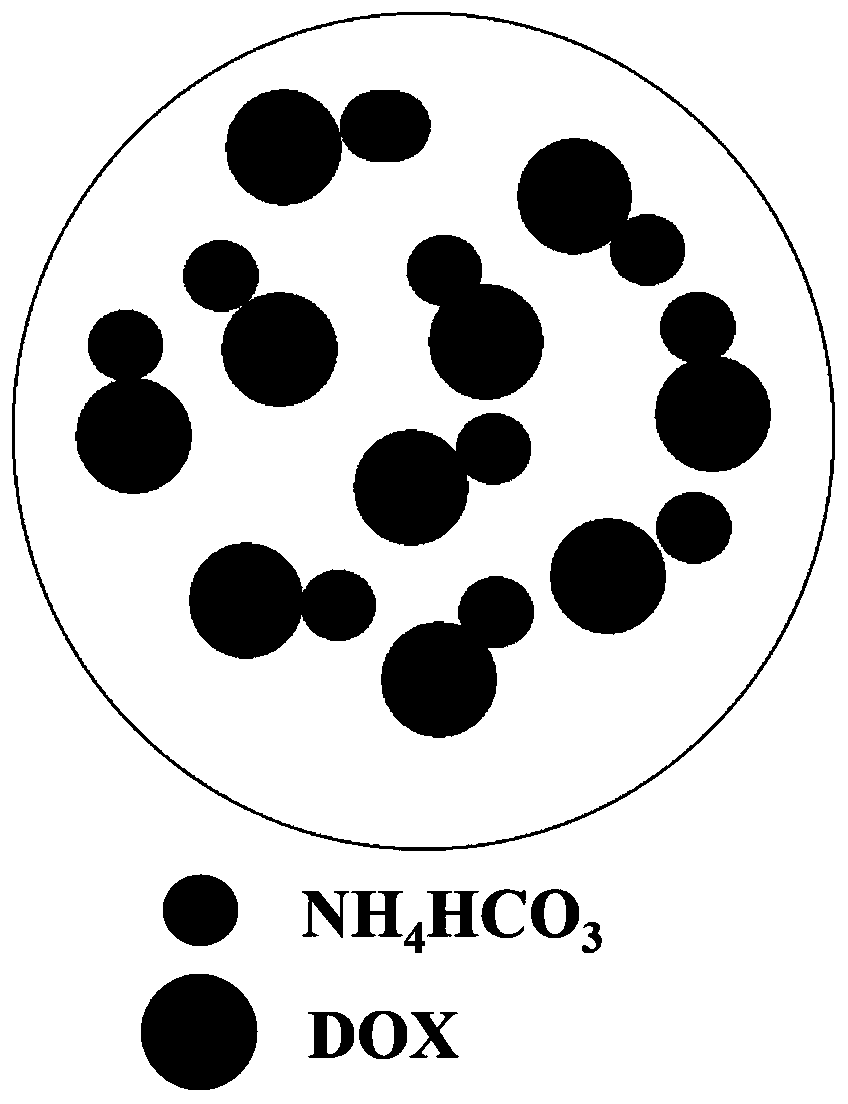
Drug-loaded hybrid nanoparticles and preparation method thereof
A technology of hybrid nanoparticles and drug loading, which is applied in the field of biomedical materials, can solve the problems of low drug loading, slow drug release, poor anti-tumor effect and safety, and achieve good biocompatibility and no toxic side effects , high safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
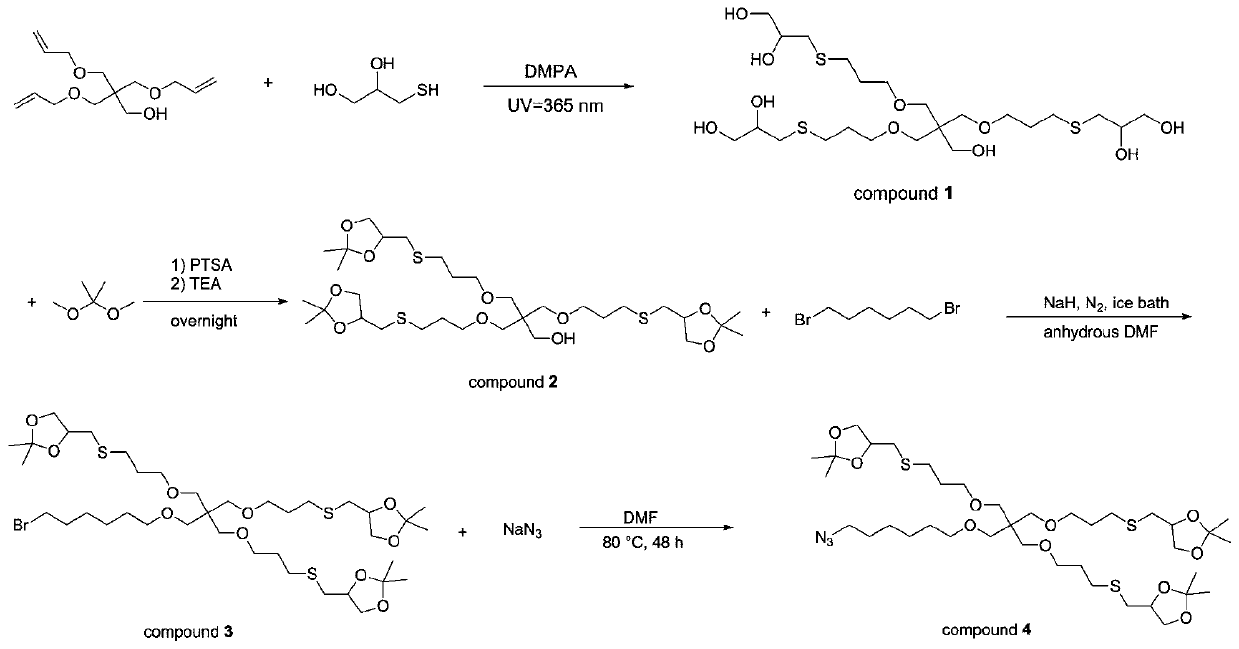
[0049] Example 1 Preparation of azide triketal compound
[0050] The schematic diagram of the preparation route of the azide triketal compound is shown in figure 2 , Specifically: Weigh a small amount of 2,2-dimethoxy-phenylacetophenone into a round bottom flask, heat it quickly outside to melt, then add pentaerythritol triallyl ether and thioglycerol, stir to dissolve Then, the reaction was stirred for 1 hour under a 365nm UV lamp, then a small amount of methanol was added to dissolve, and then a large amount of petroleum ether was added. After stirring and standing, the methanol layer was taken and the solvent was removed to obtain Intermediate 1; Intermediate 1 and 2,2-Dimethoxy Mix with propyl propane, slowly add p-toluenesulfonic acid hydrate in small amounts several times, mix and stir at 30°C-40°C for 12h, add triethylamine, continue the reaction for 0.5h, and separate by column chromatography (V Petroleum ether :V Ethyl acetate =4:1), finally remove the solvent to obta...
Embodiment 2
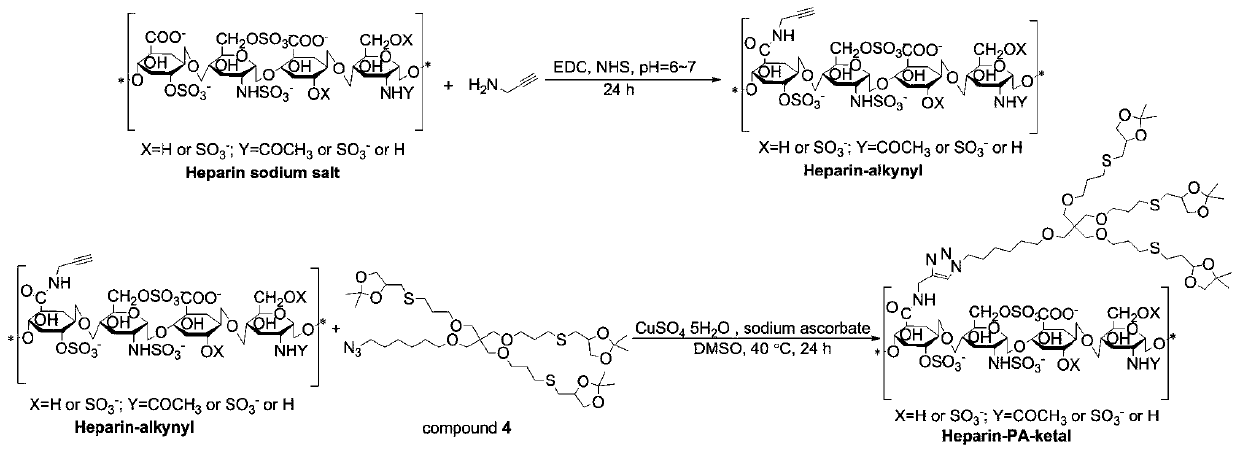
[0052] Example 2 Preparation of propargylamine modified heparin sodium
[0053] Dissolve heparin sodium, 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride and N-hydroxysuccinimide in water, add propargylamine and adjust the pH of the solution to 6 7. React at room temperature for 24 hours, dialyzed with deionized water for 72 hours and freeze-dry; among them, heparin sodium, 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride, N-hydroxysuccinyl The mass ratio of imine to propargylamine is 1:0.56:0.34:0.029.
[0054] Dissolve the resulting product in D 2 O, do 400MHz 1 H-NMR scan, see the results Figure 5 .
Embodiment 3
[0055] Example 3 Propargylamine modified heparin sodium grafted azide triketal compound
[0056] See the preparation route of propargylamine modified heparin sodium grafted azide triketal compound image 3 , Specifically: heparin sodium modified by propargylamine, azide triketal compound, copper sulfate pentahydrate and sodium ascorbate in a mass ratio of 1:1.5:0.125:0.8 miscible in DMSO, under the protection of nitrogen at 40 Reaction at ℃ for 24h, DMSO dialysis for 72h, then water dialysis for 72h to remove organic solvent, and freeze-drying.
[0057] Dissolve the resulting product in D 2 O do 400M 1 H-NMR scan, nuclear magnetic spectrum such as Image 6 Shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com