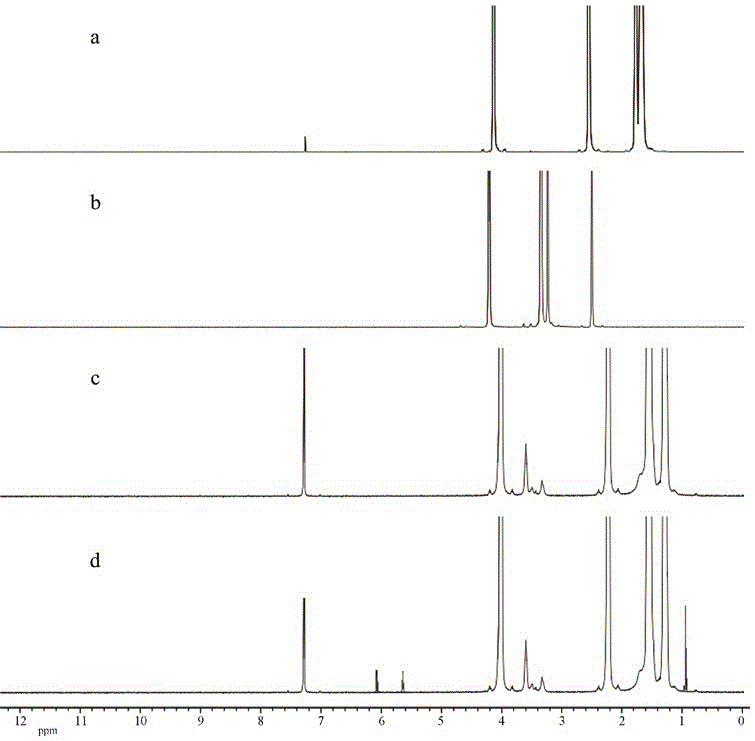
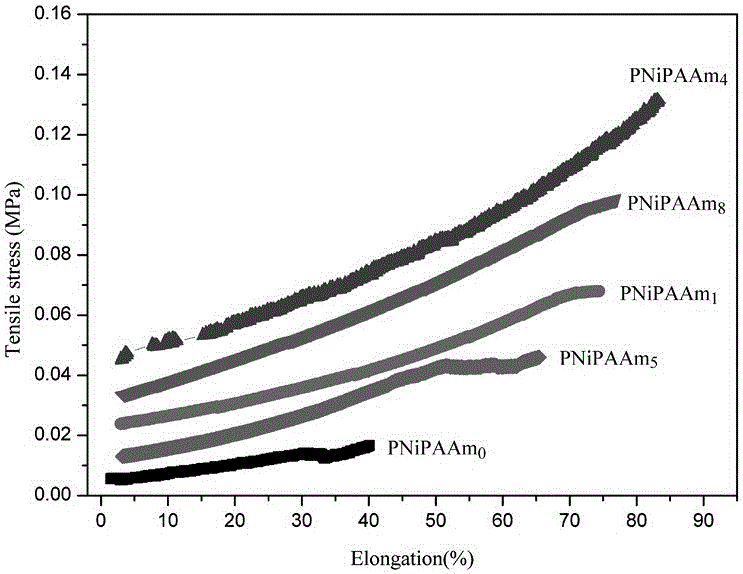
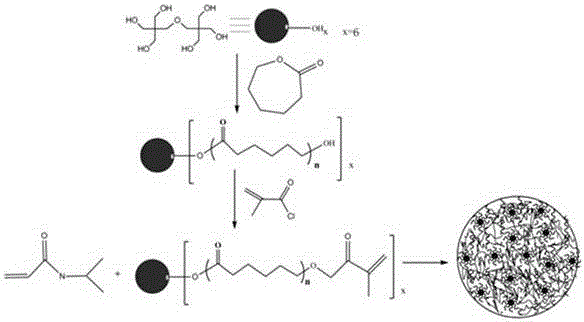
Novel poly N-isopropylacrylamide hydrogel, and preparation method and application thereof
A technology of isopropylacrylamide and methacryloyl chloride, applied in the field of smart hydrogels, can solve the problems of poor mechanical properties of PNIPAAm hydrogels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Preparation of dipentaerythritol polycaprolactate (DPCL)
[0035] Add 0.254g dipentaerythritol (DPE, 1mmol), 0.685g 6-caprolactone ( ε - CL, 60 mmol), under the protection of nitrogen, it was melted and mixed uniformly at 120 °C, and then 0.1 wt% stannous octoate was added dropwise as a catalyst, and reacted at 120 °C for 6 h under the protection of nitrogen. After the reaction is completed, cool to room temperature, dissolve with dichloromethane, then add dropwise to ice-cold n-hexane / methanol to precipitate, filter, dry, and vacuum dry;
[0036] (2) Preparation of methacrylic acid-terminated dipentaerythritol polycaprolactate (DPCLMAC)
[0037] Dissolve 0.65g (1 mmol) of DPCL in 40g of dichloromethane, add 0.55g (54 mmol) of triethylamine, stir to dissolve completely, add dropwise 0.38g of methacryloyl chloride (MAC, 36 mmol ) and 20g of dichloromethane, the dropwise addition time is controlled at 2-3h, after the dropwise addition is completed, the temperature ...
Embodiment 2
[0041] (1) Preparation of dipentaerythritol polycaprolactate (DPCL)
[0042] Add 0.254g dipentaerythritol (DPE, 1mmol), 0.685g 6-caprolactone ( ε - CL, 60 mmol), under the protection of nitrogen, it was melted and mixed uniformly at 120 °C, and then 0.1 wt% stannous octoate was added dropwise as a catalyst, and reacted at 120 °C for 6 h under the protection of nitrogen. After the reaction is completed, cool to room temperature, dissolve with dichloromethane, then add dropwise to ice-cold n-hexane / methanol to precipitate, filter, dry, and vacuum dry;
[0043] (2) Preparation of methacrylic acid-terminated dipentaerythritol polycaprolactate (DPCLMAC)
[0044] Dissolve 0.65g (1 mmol) of DPCL in 40g of dichloromethane, add 0.55g (54 mmol) of triethylamine, stir to dissolve completely, add dropwise 0.38g of methacryloyl chloride (MAC, 36 mmol ) and 20g of dichloromethane, the dropwise addition time is controlled at 2-3h, after the dropwise addition is completed, the temperature ...
Embodiment 3
[0048] (1) Preparation of dipentaerythritol polycaprolactate (DPCL)
[0049] Add 0.254g dipentaerythritol (DPE, 1mmol), 0.685g 6-caprolactone ( ε - CL, 60 mmol), under the protection of nitrogen, it was melted and mixed uniformly at 120 °C, and then 0.1 wt% stannous octoate was added dropwise as a catalyst, and reacted at 120 °C for 6 h under the protection of nitrogen. After the reaction is completed, cool to room temperature, dissolve with dichloromethane, then add dropwise to ice-cold n-hexane / methanol to precipitate, filter, dry, and vacuum dry;
[0050] (2) Preparation of methacrylic acid-terminated dipentaerythritol polycaprolactate (DPCLMAC)
[0051] Dissolve 0.65g (1 mmol) of DPCL in 40g of dichloromethane, add 0.55g (54 mmol) of triethylamine, stir to dissolve completely, add dropwise 0.38g of methacryloyl chloride (MAC, 36 mmol ) and 20g of dichloromethane, the dropwise addition time is controlled at 2-3h, after the dropwise addition is completed, the temperature ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com