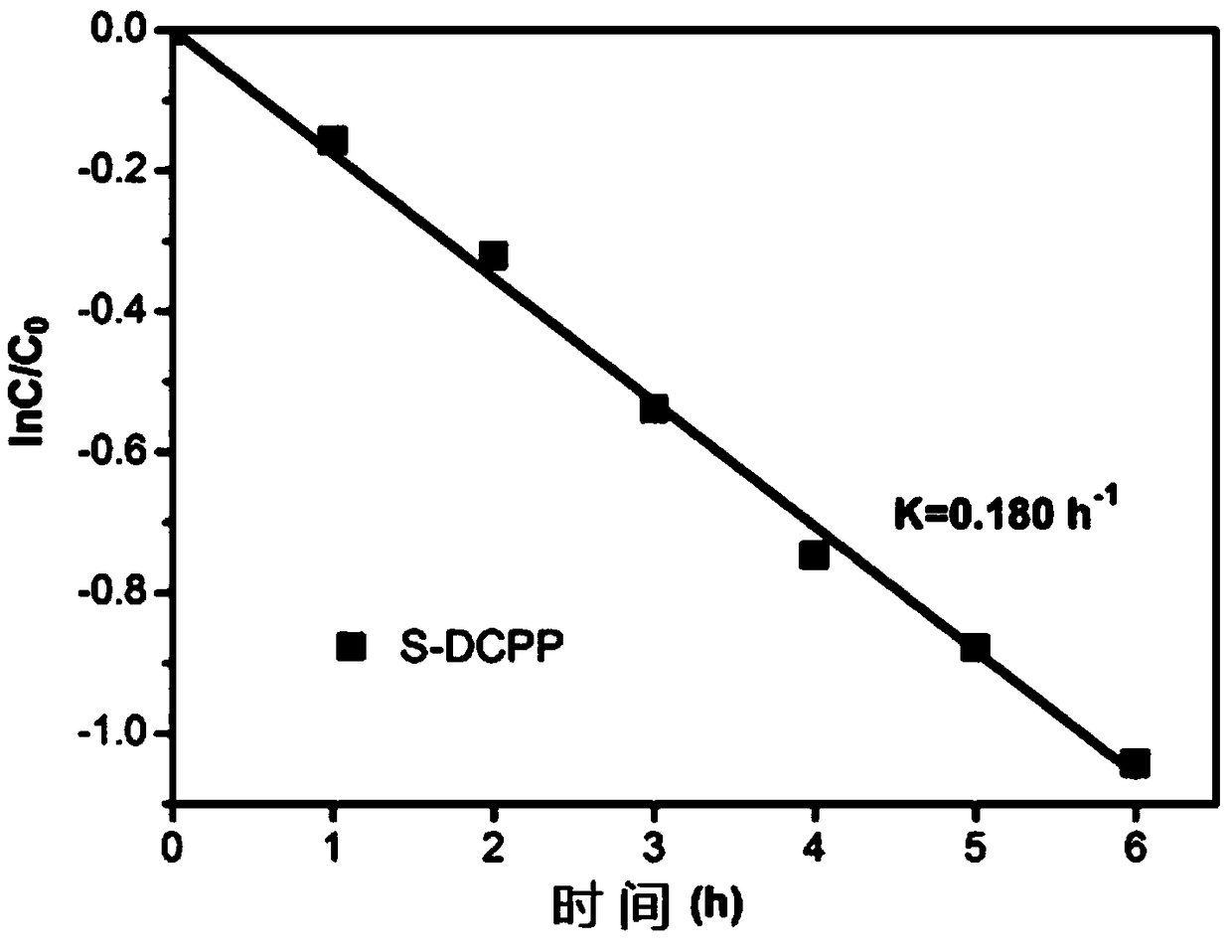
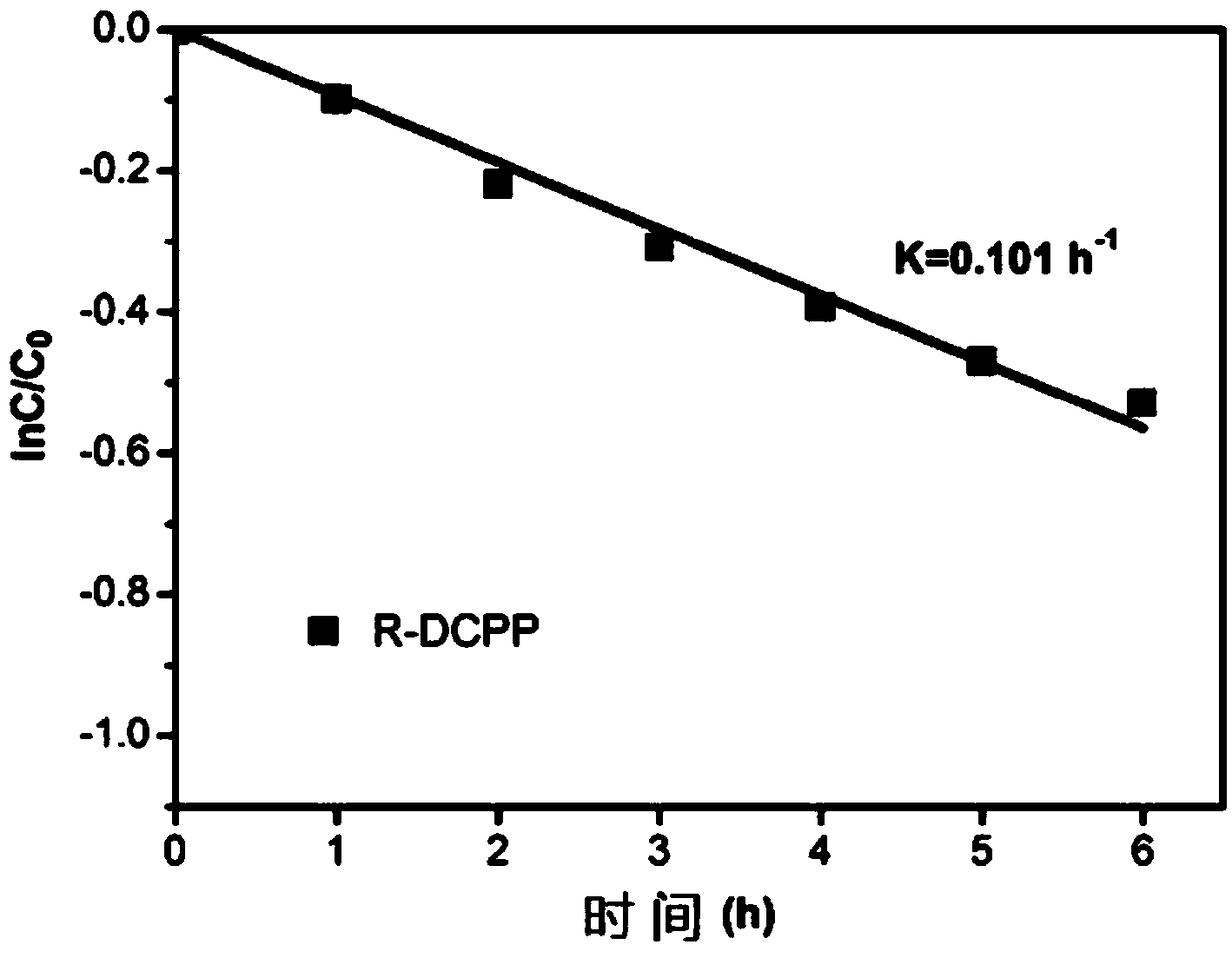
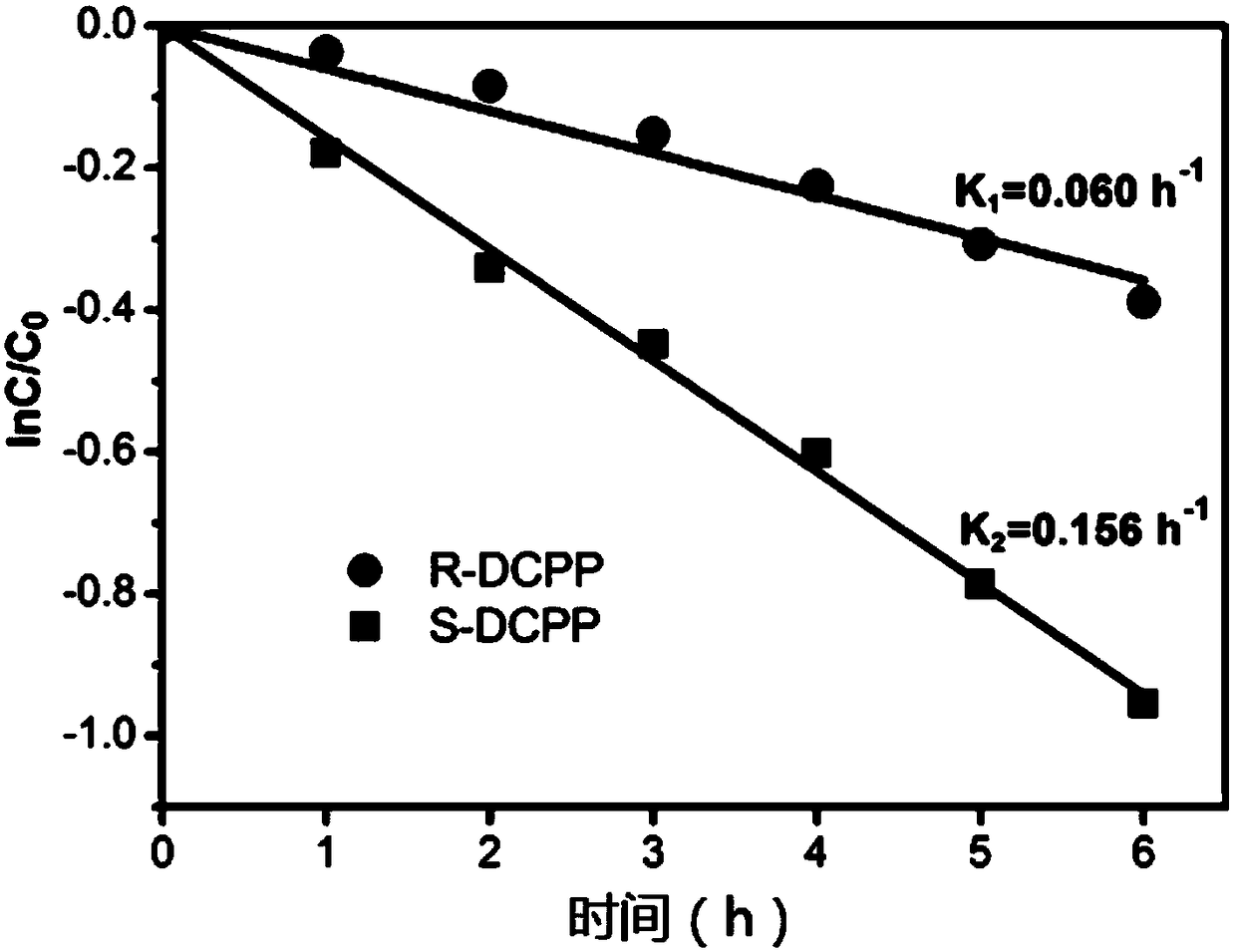
Enantioselective Photoelectrochemical Oxidative Degradation Method of Chiral Pesticide 2,4-Dipropionic Acid
An enantioselective, photoelectrochemical technology, applied in the direction of protective devices against harmful chemicals, can solve the problems of inability to achieve selective catalytic degradation, lack of selectivity, recognition, etc., and achieve clear and selective molecular imprinting sites. High performance and strong anti-interference ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] A chiral pesticide-selective photoanode comprising 1D single-crystal TiO 2 Nanorod electrodes, and imprinted on 1D single crystal TiO 2 S-2,4-DPO imprinted sites on the surface of nanorod electrodes, fabricated by the following steps:
[0038] (1) Weigh concentrated hydrochloric acid and mix it with water, then add tetrabutyl titanate dropwise, stir at 450rpm for 1h, add template molecule S-2,4-dipropionic acid, concentrated hydrochloric acid, water, tetrabutyl titanate and The volume ratio of the added amount of S-2,4-dipropionic acid is 10:10:0.25:0.05, the concentration of concentrated hydrochloric acid is 37.5wt%, and the precursor solution is obtained;
[0039] (2) Transfer the precursor solution to a sealed reaction kettle, immerse the conductive side of the pretreated FTO in the solution, and react at 150°C for 5h;
[0040](3) After the reaction is completed, take out the product, wash it with deionized water, take out the surface residue, and calcinate at 500°...
Embodiment 2
[0043] A chiral pesticide-selective photoanode comprising 1D single-crystal TiO 2 Nanorod electrodes, and imprinted on 1D single crystal TiO 2 S-2,4-DPO imprinted sites on the surface of nanorod electrodes, fabricated by the following steps:
[0044] (1) Weigh concentrated hydrochloric acid and mix it with water, then add tetrabutyl titanate dropwise, stir at 300rpm for 0.5h, add template molecule S-2,4-dipropionic acid, concentrated hydrochloric acid, water, tetrabutyl titanate The volume ratio of the added amount of S-2,4-dipropionic acid is 10:15:0.4:0.1, the concentration of concentrated hydrochloric acid is 39wt%, and the precursor solution is obtained;
[0045] (2) Transfer the precursor solution to a sealed reaction kettle, immerse the conductive side of the pretreated FTO in the solution, and react at 120°C for 8 hours;
[0046] (3) After the reaction is completed, take out the product, rinse it with deionized water, take out the surface residue, and calcinate it at ...
Embodiment 3
[0048] A chiral pesticide-selective photoanode comprising 1D single-crystal TiO 2 Nanorod electrodes, and imprinted on 1D single crystal TiO 2 S-2,4-DPO imprinted sites on the surface of nanorod electrodes, fabricated by the following steps:
[0049] (1) Weigh concentrated hydrochloric acid and mix it with water, then add tetrabutyl titanate dropwise, stir at 600rpm for 1.5h, add template molecule S-2,4-dipropionic acid, concentrated hydrochloric acid, water, tetrabutyl titanate The volume ratio of the added amount of S-2,4-dipropionic acid is 10:5:0.1:0.01, the concentration of concentrated hydrochloric acid is 36wt%, and the precursor solution is obtained;
[0050] (2) Transfer the precursor solution to a sealed reaction kettle, immerse the conductive side of the pretreated FTO in the solution, and react at 180°C for 2 hours;
[0051] (3) After the reaction is completed, take out the product, wash it with deionized water, take out the surface residue, and calcinate it at 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com