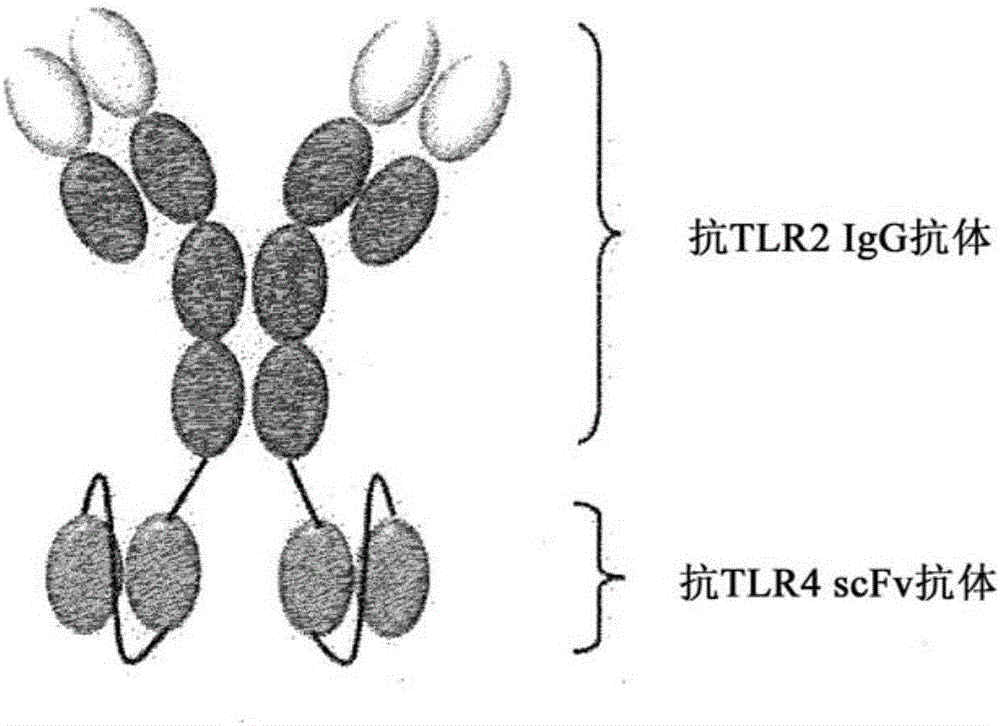
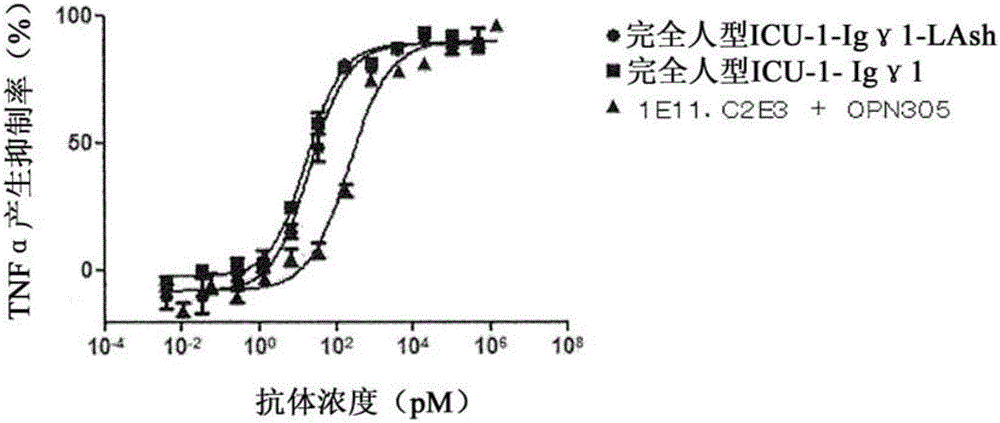
Novel bispecific antibody binding to human TLR2 and human TLR4
A bispecific antibody and antibody technology, applied in the direction of antibodies, antibody medical components, antibody mimics/scaffolds, etc., can solve the problems of bispecific antibodies that have not been reported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0264]Anti-human TLR2 antibodies were produced using human monoclonal antibody development technology "VelocImmune" (VelocImmune antibody technology; Regeneron Corporation (US Patent No. 6596541)) mice. VelocImmune mice were immunized with human TLR2 protein (R&D systems, 2616-TR-050) together with an adjuvant to induce an immune response. Lymphocytes were collected from the spleen or lymph nodes of the immunized mice according to a conventional method, and were fused with mouse myeloma cells SP2 / 0 (ATCC: CRL-1581) to produce hybridomas. Monoclonalization was carried out, and culture was carried out in CD hybridoma medium (Life Technologies Co., Ltd.), which is a serum-free medium. Antibodies were purified from the obtained culture supernatant using a Protein G column (GE ヘルスケア). In the VelocImmune technology, transgenic mice in which the variable regions of the endogenous immunoglobulin heavy and light chains are replaced by the corresponding human variable regions are used....
Embodiment 2
[0265] (Example 2: Evaluation of neutralizing activity of anti-human TLR2 antibody)
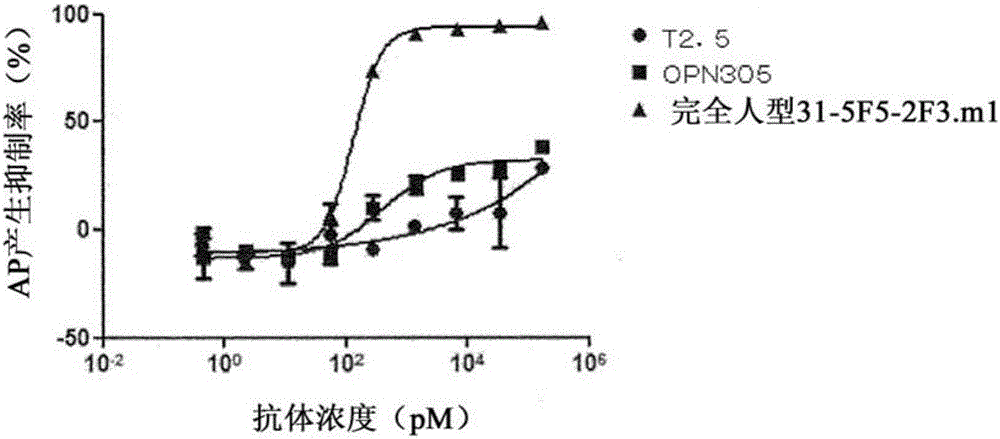
[0266] In order to evaluate the neutralizing activity of the anti-human TLR2 antibodies determined in Example 1, the human monocytic THP1-xBlue cells endogenously expressing human TLR2 were used to detect the induction of alkaline phosphatase (AP) against the TLR2 / 6 agonist Pam2CSK4. ) produced inhibition.
[0267] As a result, it was found that the anti-human TLR2 antibody (chimeric antibody) named 31-5F5-2F3 inhibited AP production and had neutralizing activity against human TLR2.
Embodiment 3
[0268] (Example 3: Sequence determination of anti-human TLR2 antibody and production of mutants)
[0269] The sequences of the genes encoding the heavy and light chains of the antibody were analyzed from the hybridoma producing the anti-human TLR2 antibody 31-5F5-2F3, and sequence determination was performed.
[0270] After the sequence of the antibody was determined, in order to improve the physical properties and stability of the antibody, the FRs of the heavy chain and light chain of 31-5F5-2F3 were replaced with FRs of other human antibodies to produce the anti-human TLR2 antibody 31-5F5-2F3.m1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com