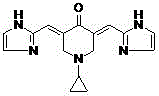
Application of 3,5-(E)-diarylmethylene-N-cyclopropyl piperidin-4-one compounds as Hsp90 depressant
A technology of cyclopropylpiperidine and ketone compounds, which is applied in the application field of 3,5-dibenzylidene-N-cyclopropylpiperidin-4-one compound, in the preparation of anti-tumor drugs, can Solve the problems of weak activity and unclear targets, and achieve the effect of strong inhibitory activity and strong proliferation inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
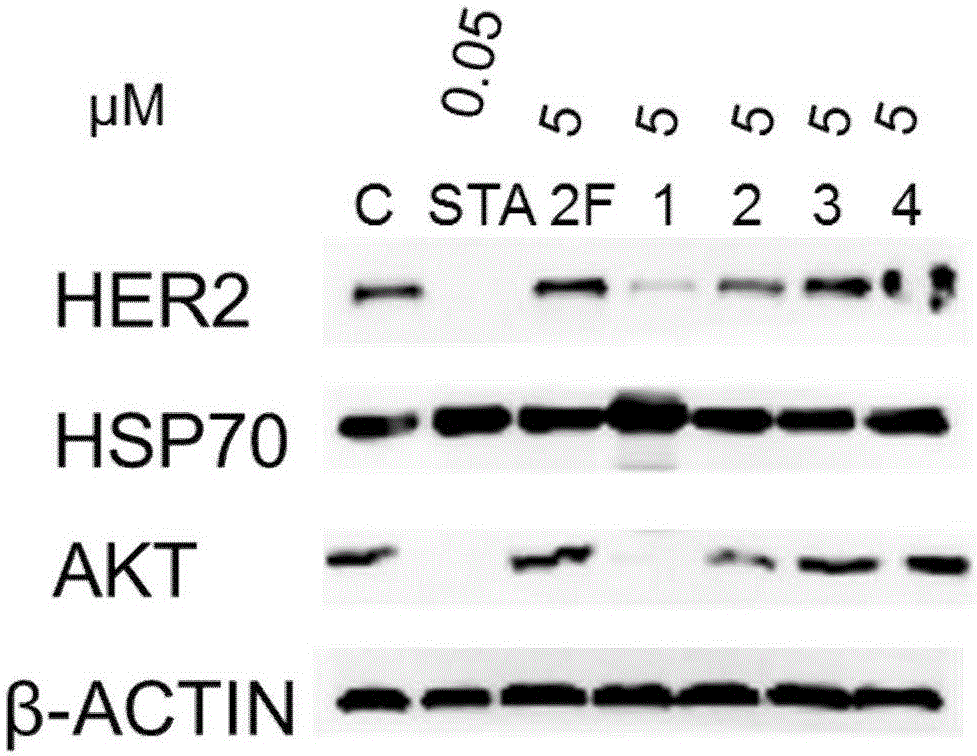
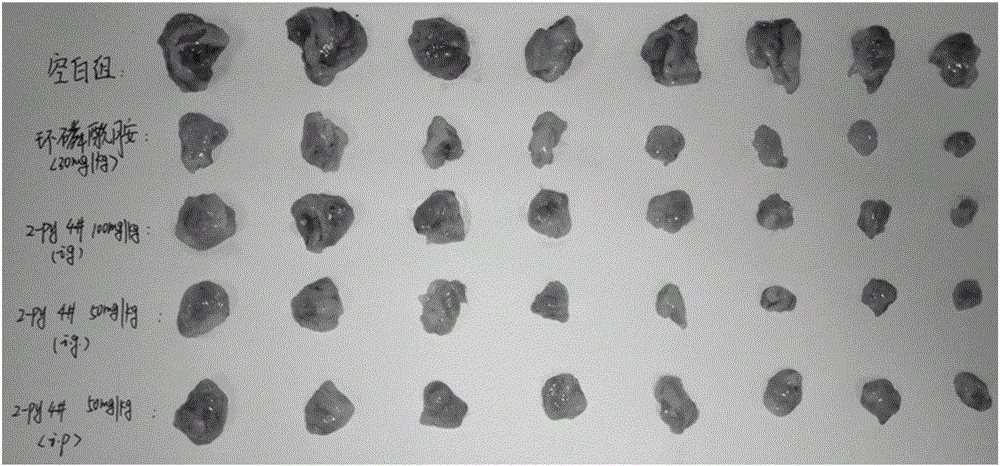
Image
Examples
Embodiment 1
[0014] 3,5-( E )- two (2-pyridine) methylene- N - Preparation of cyclopropylpiperidin-4-one (compound 1)
[0015] N -Cyclopropyl-4-piperidone 348 mg (2.5 mmol), sodium hydroxide 20 mg (0.5 mmol), absolute ethanol 20 mL, mix and stir at room temperature, add 2-pyridinecarbaldehyde 535 mg (5 mmol ), reacted at room temperature for 2h~24h, and solid precipitated out. Suction filtration, the filter cake was washed with absolute ethanol, passed through a silica gel column, eluent petroleum ether: ethyl acetate (3:1) gradient elution, to obtain 348 mg of light yellow solid, yield 47%, melting point 137-138 ° C .
[0016]
[0017] 1 H NMR (400 MHz, Chloroform- d ) δ 8.76 (d, J = 1.8 Hz, 2H), 7.75-7.70(m, 2H), 7.61 (s, 2H), 7.47 (d, J = 7.8 Hz, 2H), 7.24-7.21 (m, 2H), 4.38 (s,4H), 2.06-2.00 (m, 1H), 0.56-0.53(m, 2H), 0.48-0.46 (m, 2H). MS[M+H] + 318.2
Embodiment 2
[0019] 3,5-( E )- two (3-pyridine) methylene- N - Preparation of cyclopropylpiperidin-4-one (compound 2)
[0020]
[0021] This product consists of the aforementioned N -Cyclopropyl-4-piperidone 348 mg (2.5 mmol) and 3-pyridinecarbaldehyde 535 mg (5 mmol) were synthesized according to the method of Example 1 to obtain 79 mg of light yellow solid, yield 9%, melting point 158-160 ° c.
[0022] 1 H NMR (400 MHz, Chloroform- d ) δ 8.70 (s, 2H), 8.60 (d, J =1.6 Hz,2H), 7.74-7.71(m, 4H), 7.39 (d, J = 8.0, 2H), 3.98 (s, 4H), 1.97-1.93 (m,1H), 0.53-0.48 (m, 2H), 0.40 – 0.36 (m, 2H). MS[M+H] + 318.2
Embodiment 3
[0024] 3,5-( E )- two (4-pyridine) methylene- N - Preparation of cyclopropylpiperidin-4-one (compound 3)
[0025]
[0026] This product consists of the aforementioned N -Cyclopropyl-4-piperidone 348 mg (2.5 mmol) and 4-pyridinecarbaldehyde 535 mg (5 mmol) were synthesized according to the method of Example 1 to obtain 74 mg of light yellow solid, yield 8%, melting point 139-140 ° c.
[0027] 1 H NMR (400 MHz, Chloroform- d ) δ 8.76 – 8.63 (m, 2H), 7.65 (d, J =2.0 Hz, 1H), 7.32 (d, J = 5.6 Hz, 0H), 7.30 – 7.25 (m, 2H), 3.96 (s, 4H),1.95 -1.93(m, 1H), 0.54-0.50 (m, 2H), 0.41 – 0.37(m, 2H).
[0028] MS[M+H] + 318.2
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com