Preparation method and application of a class of baicalein-7-methyl ether derivatives
A technology of baicalein and derivatives, applied in the field of medicine, can solve the problem of low activity, and achieve the effects of easy handling, easy availability of materials and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
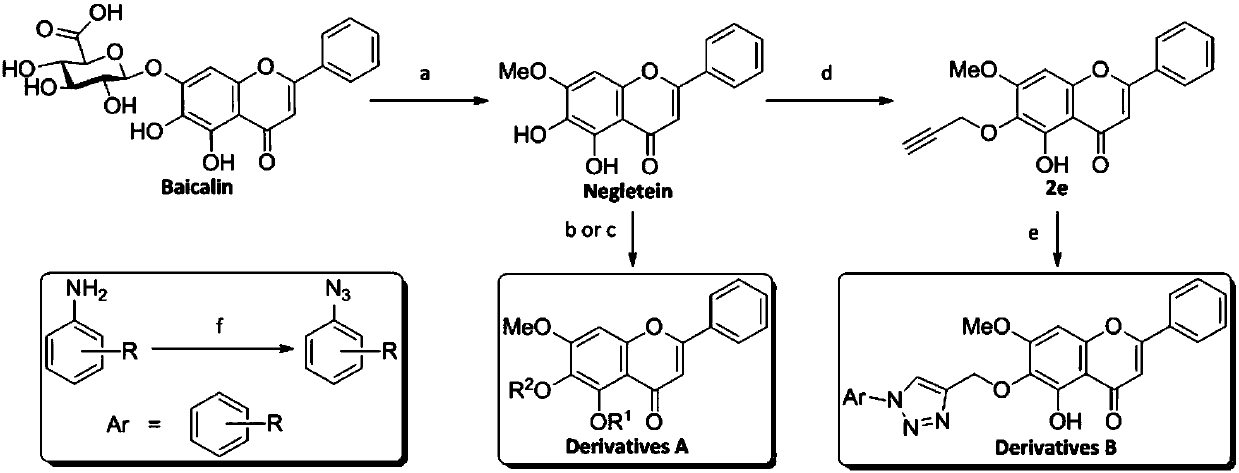
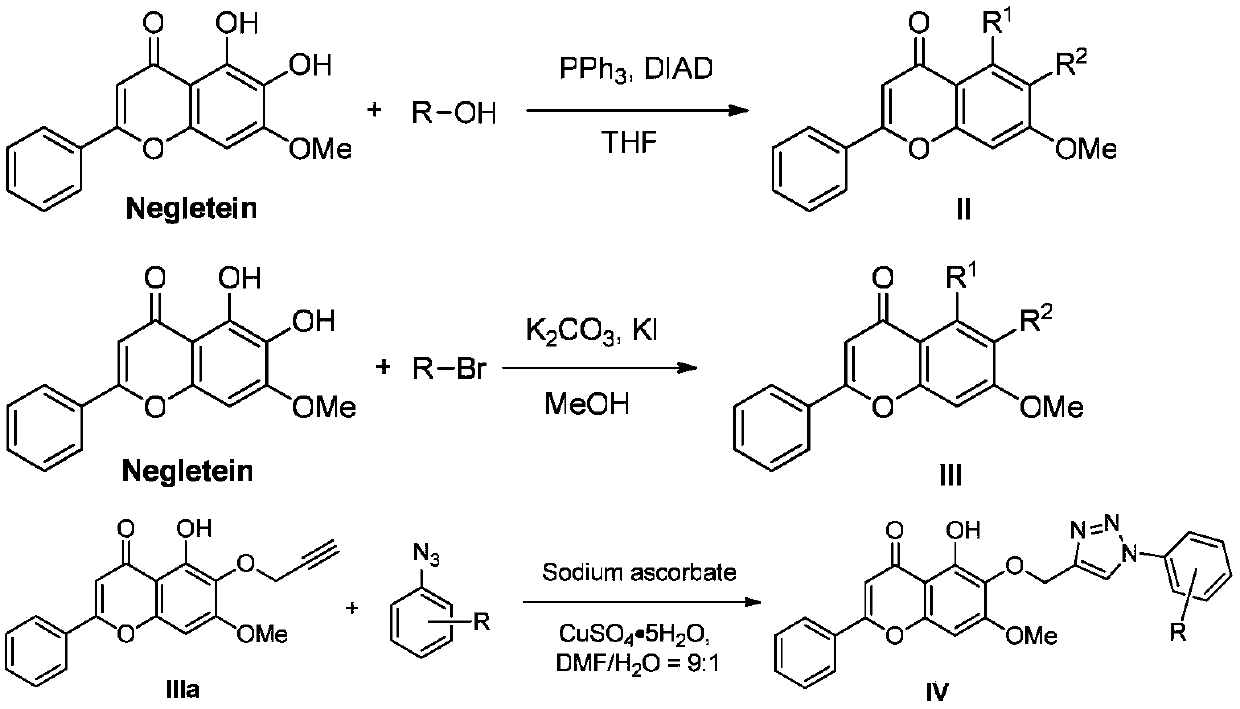
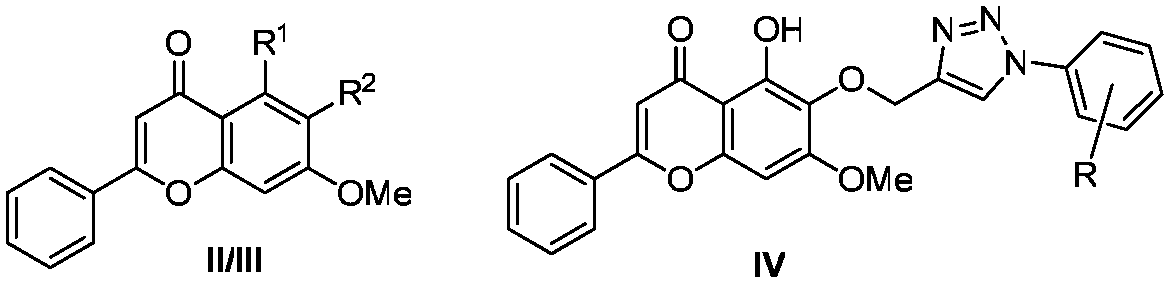
[0042] Compounds IIIb and IIIc were prepared similarly to compound IIIa, substituting 3-bromopropyne for benzyl bromide.
[0043] Compound IIIb: 1 H NMR (400MHz, CDCl 3 )δ12.73(s, 1H), 7.88–7.84(m, 2H), 7.55–7.48(m, 5H), 7.35(t, J=7.2Hz, 2H), 7.30(d, J=7.1Hz, 1H ), 6.65(s, 1H), 6.51(s, 1H), 5.12(s, 2H), 3.89(s, 3H). 13 C NMR (101MHz, CDCl 3 )δ182.79, 163.97, 159.27, 153.46, 153.34, 137.56, 131.90, 131.46, 131.37, 129.17, 128.64, 128.28, 128.07, 126.31, 106.30, 105.66, 90.69, 74.93 for ms C 23 h 19 o 5 [M+H] + 375.1227, found: 375.1226.
[0044] Compound IIIc: 1 H NMR (400MHz, CDCl 3 )δ7.89(dd, J=6.2, 2.5Hz, 2H), 7.69–7.65(m, 2H), 7.53–7.49(m, 3H), 7.47–7.43(m, 2H), 6.83(s, 1H) , 6.70(s, 1H), 5.16(s, 2H), 5.04(s, 2H), 3.94(s, 3H); 13 C NMR (101MHz, CDCl 3 )δ177.30,161.26,158.24,154.86,139.79,137.27,137.21,131.69,131.37,129.44,129.07,128.79,128.39,128.36,128.17,113.42,108.48,96.68,75.96,56.35;Melting Point:125.1-126.3℃ ;HRMS(m / z): calcd for C 30 h 25 o 5 [M+H] ...
Embodiment 1
[0057] Example 1: Inhibitory Effects of Compounds on Tumor Cell Proliferation and Survival
[0058] Material: leukemia cells HL-60.
[0059] Drugs to be tested: compounds at 5 concentrations of 0, 5 μM, 10 μM, 20 μM, and 40 μM.
[0060] The inhibitory effect of compound IIa on the proliferation and survival of leukemia cells HL-60: After HL-60 cells were cultured, the compounds were added and incubated for 48 hours, the proliferation ability of HL-60 cells decreased, and the cells showed apoptosis changes. The results of MTT analysis showed that the survival rate of HL-60 cells showed a downward trend with the increase of IIa concentration (0, 5 μM, 10 μM, 20 μM, 40 μM), and dropped to nearly 70% at 7.24 μM.
Embodiment 2
[0061] Example 2: Effects of Compounds on the Activation of Leukemia Cell Signaling Pathways
[0062] Material: leukemia cells HL-60;
[0063] Drugs to be tested: 4 concentrations of compounds of 0, 5 μM, 10 μM, and 20 μM.
[0064] Effects on the signaling pathway of HL-60 cells: After treating HL-60 cells with IIa for 24 hours, the total protein was extracted and separated by SDS gel electrophoresis. The results of Western blot analysis showed that compound IIa inhibited the activation of Akt signaling pathway in a dose-dependent manner. And activate Caspase-3, thereby inhibiting the proliferation of HL-60 cells and promoting the apoptosis of HL-60 cells.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com