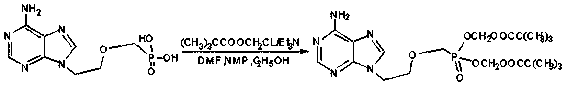A kind of synthetic technique of adefovir dipivoxil
A technology of adefovir dipivoxil and synthesis process, which is applied in the field of adefovir dipivoxil synthesis process, can solve the problems of complex process, complicated operation, low yield and the like, achieves improvement of reaction yield, simplification of production process, improved Yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] A synthetic technique for adefovir dipivoxil, comprising the following steps;
[0025] (1) In a clean glass kettle, first add 5L DMF, 5L N-methylpyrrolidone, 0.5L absolute ethanol, 1kgPMEA and 4.5kg chloromethyl pivalate, stir to raise the temperature to 60-70℃, add dropwise 3kg Triethylamine was added dropwise within 1 h, and after another 2.5 hours of reaction, the reaction was detected by HPLC. The amount of PMEA in the reaction solution was less than 0.5%, and the reaction was completed.
[0026] (2) Add 10L of ethyl acetate to the reaction solution, stir and dissolve at room temperature for 1 hour, filter, and wash the filter cake with 10L of ethyl acetate until white, and wash the obtained filtrate with 10L of 20% sodium chloride solution, discard the washing solution The water phase and the organic phase were dried with 1 kg of anhydrous calcium chloride for 2 h, filtered, and the calcium chloride solid was removed to obtain the filtrate.
[0027] (3) After conc...
Embodiment 2
[0029] A synthetic technique for adefovir dipivoxil, comprising the following steps;
[0030] (1) In a clean glass kettle, first add 5L DMF, 5L N-methylpyrrolidone, 0.5L absolute ethanol, 1kg PMEA and 5kg chloromethyl pivalate, stir to raise the temperature to 60-70°C, add dropwise 3kg Ethylamine was added dropwise within 1 h, and after another 3 hours of reaction, the reaction was detected by HPLC. The amount of PMEA in the reaction solution was less than 0.5%, and the reaction ended.
[0031] (2) Add 12L of ethyl acetate to the reaction liquid, stir and dissolve at room temperature for 1 hour, filter, and wash the filter cake with 12L of ethyl acetate until white, and wash the obtained filtrate with 12L of 20% sodium chloride solution, discard the washing liquid The water phase and the organic phase were dried with 1 kg of anhydrous calcium chloride for 2 h, filtered, and the calcium chloride solid was removed to obtain the filtrate.
[0032] (3) Concentrate the above filtr...
Embodiment 3
[0034] A synthetic technique for adefovir dipivoxil, comprising the following steps;
[0035] (1) In a clean glass kettle, first add 5L DMF, 8L N-methylpyrrolidone, 0.5L absolute ethanol, 1kgPMEA and 4.8kg chloromethyl pivalate, stir and heat up to 60-70°C, add 3kg Triethylamine was added dropwise within 1 h, and then reacted for 2 hours, and the reaction was detected by HPLC. The amount of PMEA in the reaction solution was less than 0.5%, and the reaction ended.
[0036] (2) Add 12L of ethyl acetate to the reaction solution, stir and dissolve at room temperature for 1 hour, filter, and wash the filter cake with 10L of ethyl acetate until white, and wash the obtained filtrate with 10L of 20% sodium chloride solution, discard the washing solution The water phase and the organic phase were dried with 1 kg of anhydrous calcium chloride for 2 h, filtered, and the calcium chloride solid was removed to obtain the filtrate.
[0037] (3) Concentrate the above filtrate under reduced p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com