2-carbonl-2-phenyl acetic acid p-methyl benzoyl hydrazone di-n-butyltin complex and preparation method and application thereof
A technology of di-n-butyltin and di-n-butyltin oxide with toluylhydrazone, which is applied in the fields of tin organic compounds, pharmaceutical formulations, organic chemical methods, etc., can solve the problems of undiscovered compounds and achieve simple preparation methods, High cancer activity and good anticancer activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Preparation of 2-carbonyl-2-phenylacetic acid p-toluylhydrazone di-n-butyltin complex:
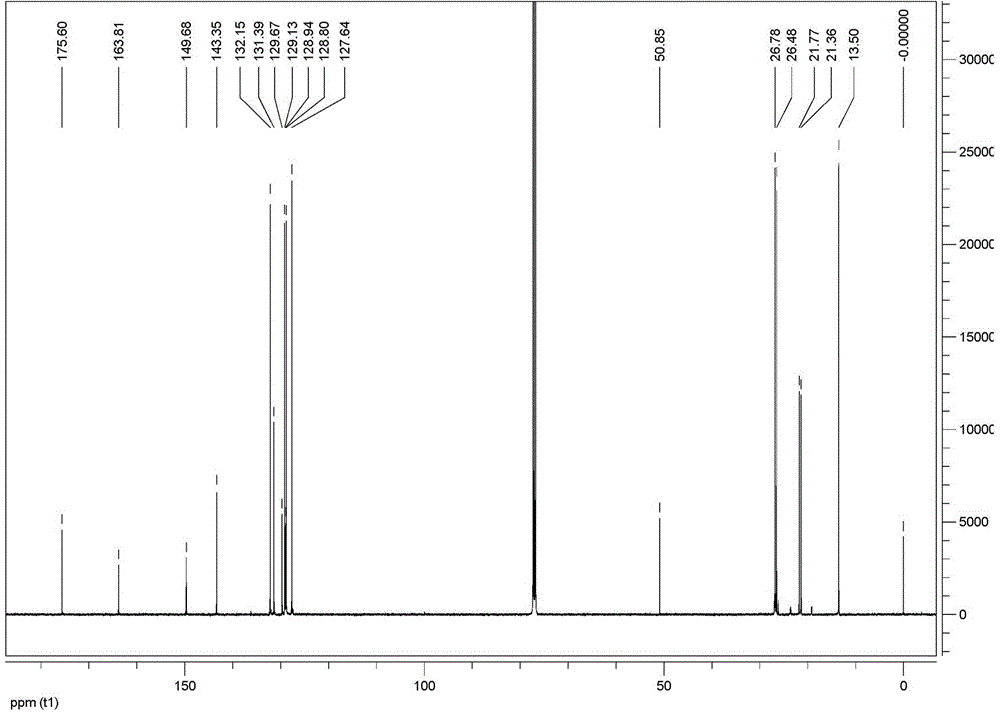
[0043] Add 0.249g (1.0mmol) di-n-butyltin oxide, 0.150g (1.0mmol) p-toluylhydrazide, 0.165g (1.1mmol) benzoylformic acid and 15mL solvent into a 100mL three-necked flask protected by nitrogen Anhydrous methanol, react at a temperature of 45~65°C for 8 hours, cool, filter, and control solvent volatilization and crystallization at a temperature of 20~35°C to obtain a yellow transparent crystal, which is 2-carbonyl-2-phenyl p-Tolylhydrazone di-n-butyltin acetate complex. Yield: 79.6%. Melting point: 98~100°C (dec).
[0044] Elemental analysis (C 50 h 68 N 4 o 8 sn 2 ): Calculated: C 55.07, H 6.29, N 5.14; Found: C 55.09, H 6.24, N 5.19.
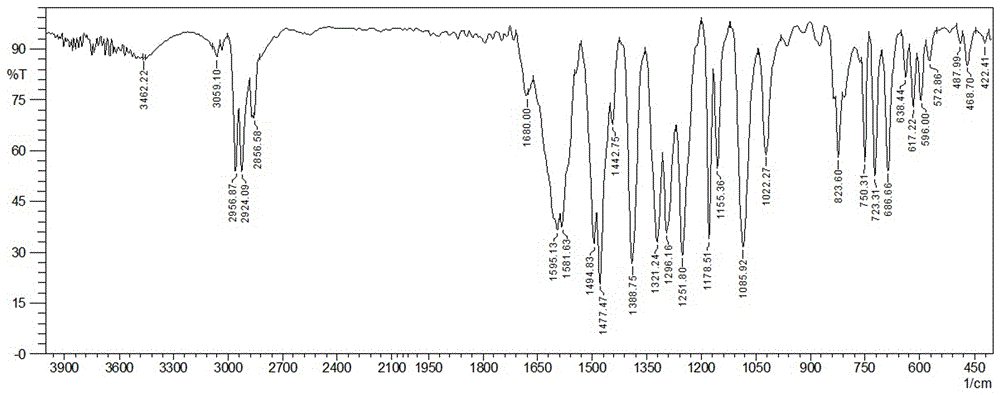
[0045] FT-IR (KBr, ν / cm -1 ): 3462, 3059, 2956, 2924, 2856, 1680, 1595, 1581, 1494, 1477, 1388, 1321, 1296, 1251, 1178, 1085, 1022, 823, 750, 5 223, 7, 96, 638 487, 468, 422.
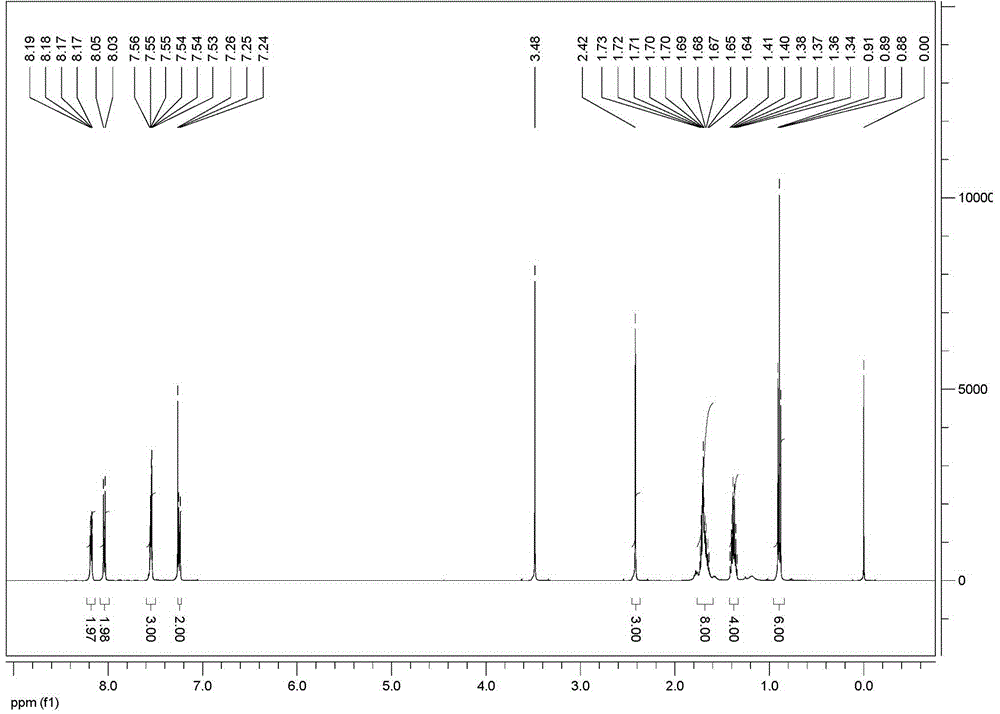
[0046] 1 H NMR (500 MHz, CDCl 3 , δ / ppm): 8.17-8.19 (m, 2H), ...
Embodiment 2
[0051] Preparation of 2-carbonyl-2-phenylacetic acid p-toluylhydrazone di-n-butyltin complex:
[0052] Add 0.249g (1.0mmol) di-n-butyltin oxide, 0.150g (1.0mmol) p-toluylhydrazide, 0.157g (1.05mmol) benzoylformic acid and 35mL solvent into a 100mL three-necked flask protected by nitrogen Anhydrous methanol, react for 5 hours at a temperature of 45~65°C, cool, filter, and control solvent volatilization and crystallization at 20~35°C to obtain a yellow transparent crystal, which is 2-carbonyl-2-phenyl p-Tolylhydrazone di-n-butyltin acetate complex. Yield: 82.5%. Melting point: 98~100°C (dec).
[0053] Elemental analysis (C 50 h 68 N 4 o 8 sn 2 ): Calculated: C 55.07, H 6.29, N 5.14; Found: C 55.09, H 6.24, N 5.19.
[0054] FT-IR (KBr, ν / cm -1 ): 3462, 3059, 2956, 2924, 2856, 1680, 1595, 1581, 1494, 1477, 1388, 1321, 1296, 1251, 1178, 1085, 1022, 823, 750, 5 223, 7, 96, 638 487, 468, 422.
[0055] 1 H NMR (500 MHz, CDCl 3 , δ / ppm): 8.17-8.19 (m, 2H), 8.04 (d, J = 8...
Embodiment 3
[0060] Preparation of 2-carbonyl-2-phenylacetic acid p-toluylhydrazone di-n-butyltin complex:
[0061] Add 0.249g (1.0mmol) di-n-butyltin oxide, 0.157g (1.05mmol) p-toluylhydrazide, 0.173g (1.15mmol) benzoylformic acid and 25mL solvent into a 100mL three-necked flask protected by nitrogen Anhydrous methanol, react for 24 hours at a temperature of 45~65°C, cool, filter, and control solvent volatilization and crystallization at a temperature of 20~35°C to obtain a yellow transparent crystal, which is 2-carbonyl-2-phenyl p-Tolylhydrazone di-n-butyltin acetate complex. Yield: 81.0%. Melting point: 98~100°C (dec).
[0062] Elemental analysis (C 50 h 68 N 4 o 8 sn 2 ): Calculated: C 55.07, H 6.29, N 5.14; Found: C 55.09, H 6.24, N 5.19.
[0063] FT-IR (KBr, ν / cm -1 ): 3462, 3059, 2956, 2924, 2856, 1680, 1595, 1581, 1494, 1477, 1388, 1321, 1296, 1251, 1178, 1085, 1022, 823, 750, 5 223, 7, 96, 638 487, 468, 422.
[0064] 1 H NMR (500 MHz, CDCl 3 , δ / ppm): 8.17-8.19 (m, 2H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com