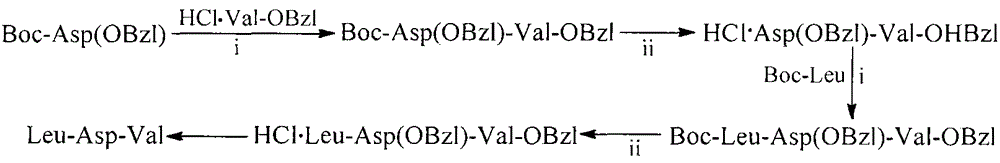Leu-Asp-Val modified curcumin, preparation method, biological activity and application thereof
A technology of -the-lys-leu-asp-val, curcumin, applied in drug combination, non-central analgesic, bulk chemical production, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Preparation of (E)-6-(4-hydroxyl-3-methoxyphenyl)hex-5-ene-2,4-dione
[0029] Dissolve 30 mL (100.0 mmol) of acetylacetone in 200 mL of ethyl acetate, and heat and stir at 60°C. While stirring, add 12.6g (200mmol) boric anhydride (B 2 o 3 ) and stirred for 1 hour. Then 27mL (100.0mmol) tri-n-butyl borate and 15.6g (100.0mmol) vanillin were added, and heated to 70°C for 30min. Afterwards, 10 mL of n-butylamine was diluted to 100 mL with ethyl acetate and slowly added to the reaction and heated to 100° C. for 2 hours. After the reaction was completed, the temperature was lowered to 60°C and 100 mL of 2N hydrochloric acid was added and stirred for 30 minutes. After the precipitate is fully separated, filter the precipitate and put it in a separatory funnel. Wash three times with saturated potassium bisulfate and saturated sodium chloride respectively. Ethyl acetate layer with anhydrous Na 2 SO 4 Let dry for 12 hours. After filtration, the filtrate was co...
Embodiment 2
[0030] Example 2 Preparation of benzyl-2-(4-formyl-2-methoxyphenoxy) ethyl acetate
[0031] Dissolve 15.6g (100.0mmol) of vanillin in 100mL of anhydrous tetrahydrofuran, add 8.2g (60mmol) of potassium carbonate and stir for 2 hours. Subsequently, 17.4 mL (120 mol) of benzyl bromoacetate was added, and the reaction was heated at 60° C. for 3 days. After the raw material disappeared, the insoluble matter was filtered off, and the filtrate was concentrated under reduced pressure to remove THF. The residue was crystallized from diethyl ether to afford 20.6 g (69%) of the title compound as a colorless solid. ESI + -MS(m / e): 301[M+H] + .
Embodiment 3
[0032]Example 3 Preparation of Benzyl 2-{4-[(1E,6E)-7-(4-Hydroxy-3-methoxyphenyl)-3,5-dioxohepta-1,6-diene-1 -yl]-2-methoxyphenoxy}ethyl acetate
[0033] Dissolve 10 g (42.7 mmol) of (E)-6-(4-hydroxy-3-methoxyphenyl)hex-5-ene-2,4-dione in 100 mL of ethyl acetate, and heat and stir at 60°C. While stirring, add 5.4g (85.4mmol) boric anhydride (B 2 o 3 ), stirred for 1 hour. Then 12mL (42.7mmol) of tri-n-butyl borate and 6.7g (42.7mmol) of ethyl benzyl-2-(4-formyl-2-methoxyphenoxy)acetate were added, and the reaction was heated at 70°C for 30min. After that, a solution of 4.2 mL of n-butylamine and 50 mL of ethyl acetate was added slowly. The temperature was raised to 100° C. for 2 hours. After the reaction was completed, the temperature was lowered to 60°C, 42mL of 2N hydrochloric acid was added, and stirred for 30 minutes. After the precipitate was fully separated, the precipitate was filtered off. The filtrate was washed three times with saturated potassium bisulfate an...
PUM
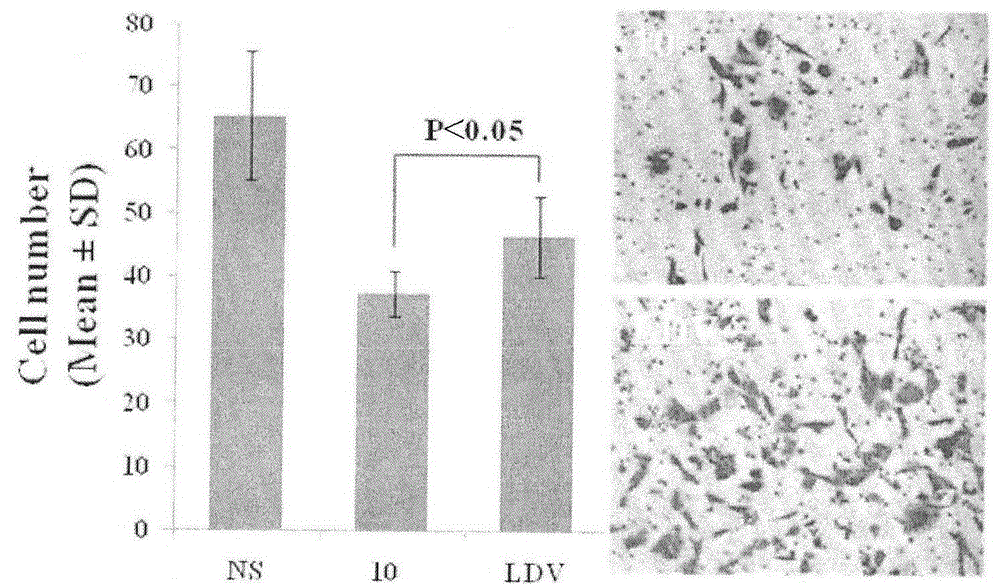
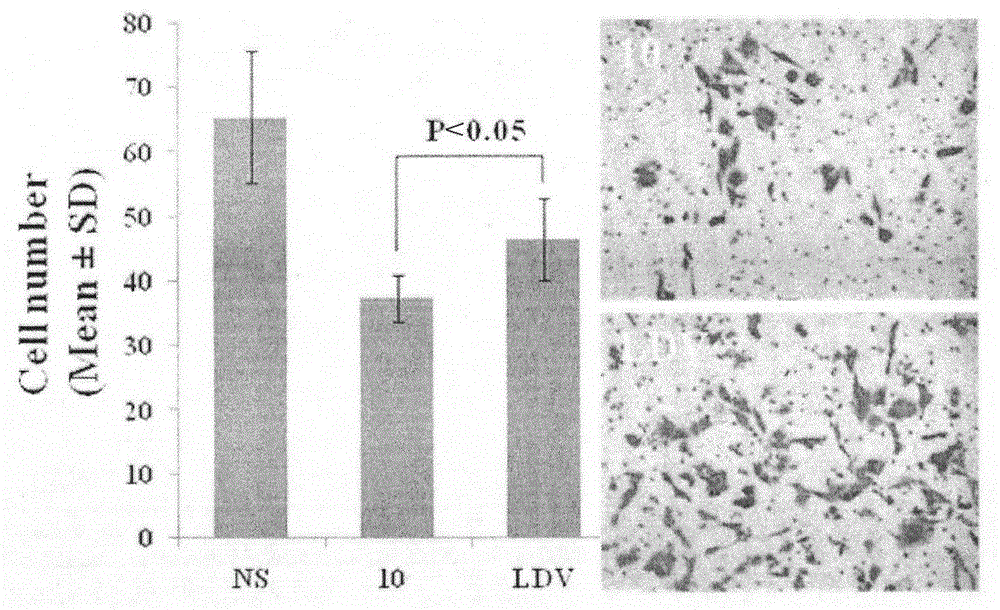
| Property | Measurement | Unit |
|---|---|---|
| Cell number | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com