Building method of Drosophila melanogaster model for screening and researching mitochondria disease virulence gene, and application of same
A genotype, mitochondrial technology, applied in biochemical equipment and methods, microbial assay/inspection, measurement devices, etc., can solve problems such as hindering the application of transgenic mouse models, long life cycle and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
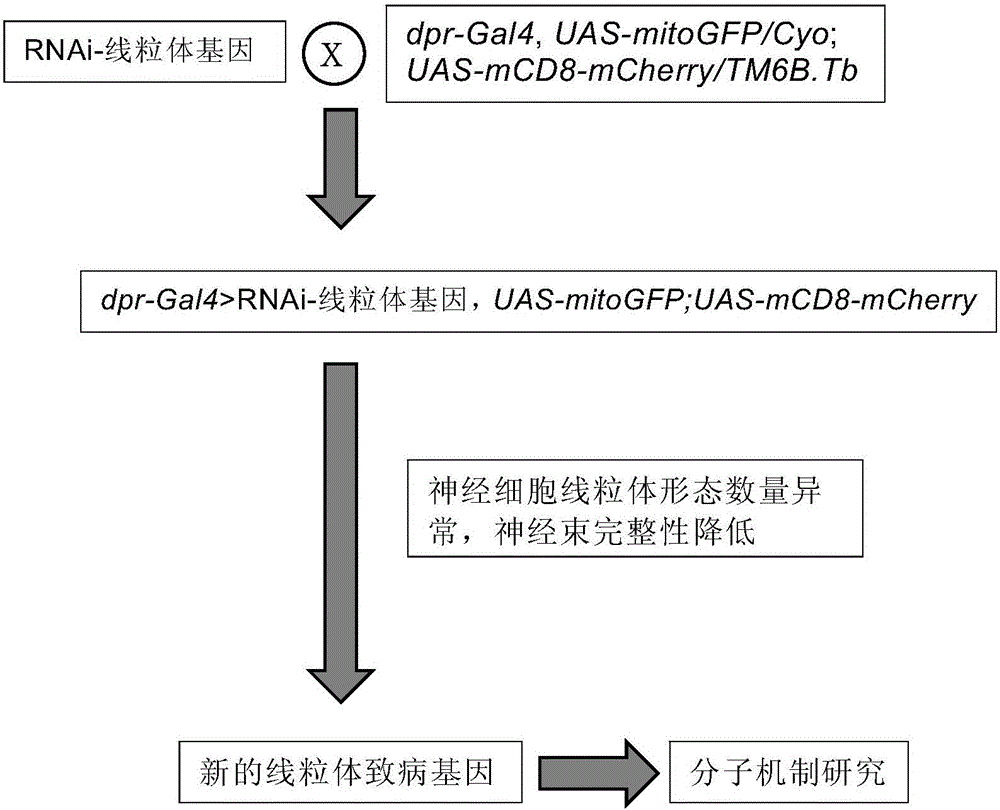
[0066] Implementation Example 1: Mitochondrial disease-causing gene screening and research establishment of Drosophila model
[0067] Cross dpr-Gal4 (chromosome II) and UAS-mitoGFP (chromosome II) two genotypes of fruit flies, and then select virgin fruit flies containing both transgenes in the progeny, and this virgin fruit fly fruit flies with w 1118 The fruit flies are hybridized, and after the hybridization, the male fruit flies with the darkest eyes are selected among the offspring fruit flies, and then these male fruit flies are separately hybridized with Sco / Cyo (chromosome II) fruit flies, and after the success of the hybridization is confirmed The existence of dpr-Gal4 and UAS-mitoGFP were verified by PCR in male fruit flies, and if the above two transgenes existed at the same time, the offspring of the fruit flies were collected, thus successfully constructing new fruit flies with fluorescent protein-labeled nerve cell mitochondria Strain dpr-Gal4, UAS-mitoGFP / Cyo; ...
Embodiment 2
[0068] Implementation Example 2: Screening of Mitochondrial Disease-causing Genes Using Mitochondrial Disease Research Model
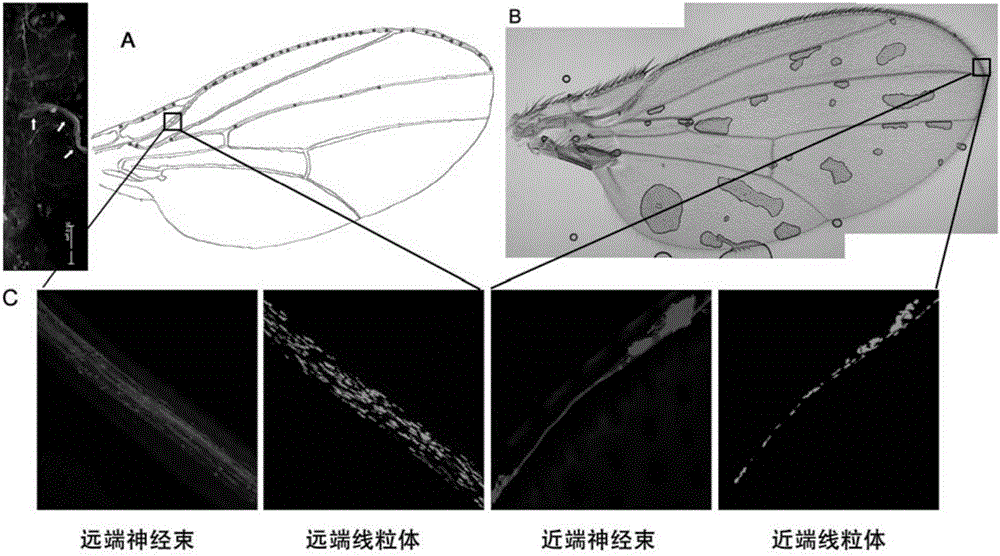
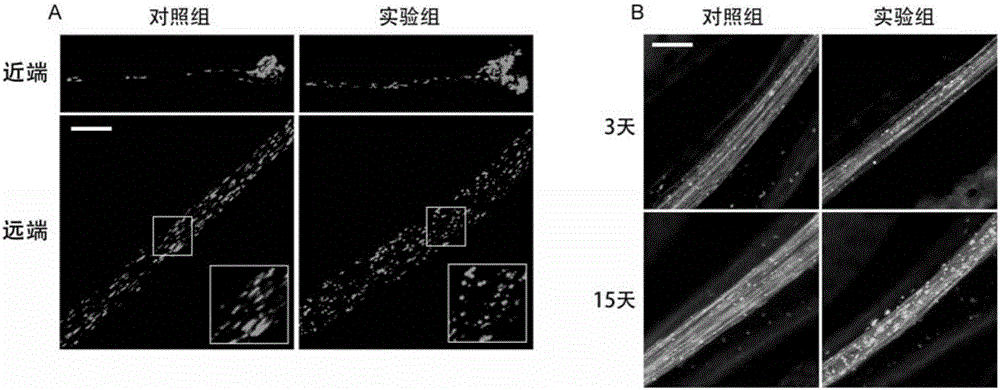
[0069] The characteristics of this screening model are that the shape and quantity of mitochondria in nerve cells are very clear, and the nerve bundles are intact. Through genetic screening, find genes that can disrupt the normal shape and quantity of mitochondria in nerve cells or the integrity of nerve bundles. Its specific implementation process is as follows figure 2 shown. The control fruit flies carrying the RNAi gene were purchased from the Drosophila Strain Center in Bloomington, USA, and the experimental group fruit flies carrying the RNAi gene were purchased from the Drosophila Strain Center of Tsinghua University.
[0070] Specific results are shown in image 3 . Part A is the morphology of the proximal and distal mitochondria of nerve cells in the control group and the experimental group. It can be seen from the figure that the mitocho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com