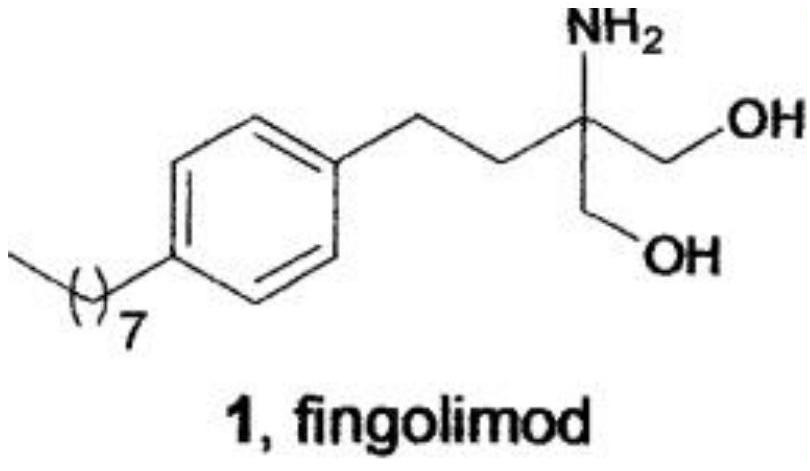
A New Synthetic Method for Fingolimod
A new synthesis and compound technology, applied in the field of new synthesis of the multiple sclerosis drug fingolimod, which can solve the problems of serious pollution, long reaction route, and potential safety hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
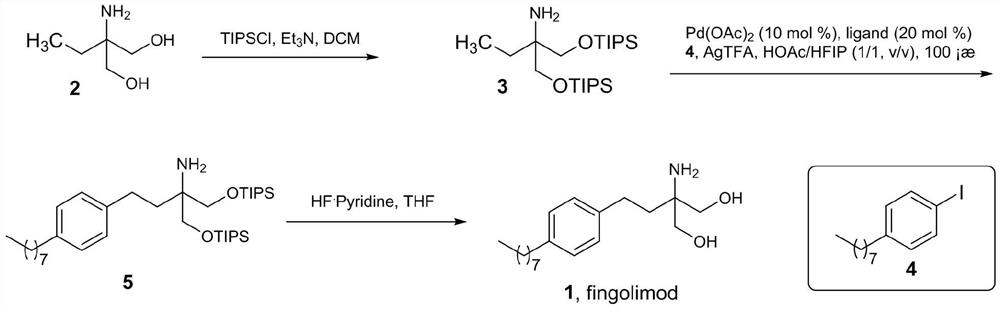
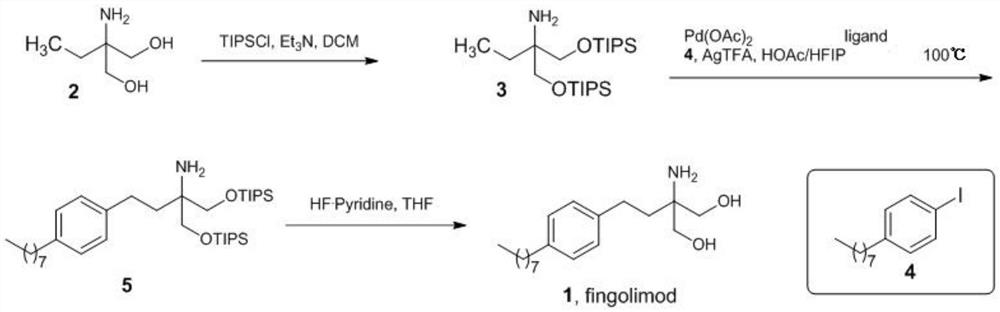
[0024] Under nitrogen protection, triisopropylchlorosilane (TIPSCl, 4.7 mL, 22mmol). After dropping, the temperature was raised to room temperature for 36 hours of reaction. The reaction was quenched with saturated ammonium chloride, and the organic phase was separated by liquid separation. The organic phase was dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to obtain 4.722 g of compound 3. The reaction product was directly used in the next reaction without purification.
[0025] Two, the synthesis of compound 5
Embodiment 2
[0027] Add palladium acetate (6.7mg, 0.03mmol), glyoxylic acid (4.44mg, 0.06mmol), silver trifluoroacetate (99.4mg, 0.45mmol), acetic acid (1mL), hexafluoroisopropanol (1mL) into the reaction flask ), compound 3 (142 mg) and compound 4 (104 mg, 0.45 mmol). After stirring and reacting at room temperature for 15 minutes in an airtight chamber, the temperature of the reaction solution was raised to 100° C. for 15 hours. After the reaction was completed, the reaction liquid was cooled to room temperature, and the reaction liquid was concentrated under reduced pressure. The concentrated solution was added with 0.3 M aqueous sodium hydroxide solution (10 mL), extracted with dichloromethane, separated, the organic phase was dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain compound 5 as a brown oil. The product was directly used in the next reaction.
[0028] 3. Synthesis of Compound 1
Embodiment 3
[0030] Compound 5 obtained in the previous step reaction was dissolved in tetrahydrofuran (8 mL) at 0° C., pyridine hydrofluoride (2 mL) (70%) was added dropwise to the solution, stirred for 1 hour and then warmed to room temperature for 20 hours. After the reaction was completed, 20 mL of an aqueous solution in which 10 g of sodium carbonate was dissolved was added dropwise to the reaction solution at 0°C. Extract with ethyl acetate (2×50 mL), separate the layers, and dry the organic phase over anhydrous sodium sulfate. Concentrate under reduced pressure, and the concentrate is treated with 10 mL of ether and 8 mL of 0.5M hydrochloric acid. The layers were separated, the organic phase was washed with 0.5M hydrochloric acid (3×8 mL), and the aqueous phases were combined. The aqueous phase was adjusted to a pH greater than 12 with sodium hydroxide. The aqueous phase was extracted with ethyl acetate (2×50 mL), the layers were separated, and the organic phase was dried over anh...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap