Preparation method of fenbendazole which is benzimidazole anti-helminthic drug
A technology of medicine benthiimazole and benzimidazole, which is applied in the field of preparation of antiparasitic raw material benthiimazole, can solve the problems of difficult operation, high cost, large environmental impact and the like, and achieves the effect of safe operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
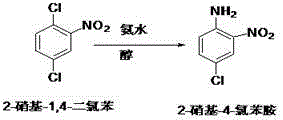
[0030] Step 1: Preparation of 2-nitro-1,4-dichlorobenzene
[0031] In a 500ml four-necked flask with a thermometer and a stirring device, start the stirring, add 120.0g of p-dichlorobenzene, 0.8 times the weight ratio of concentrated sulfuric acid, and add 1.04 times the molar amount of dichlorobenzene to nitric acid, and control the temperature of the drop. After the dripping is completed at 20~25℃, keep the reaction at 35~40℃ for 1 hour, GC will track the reaction to complete, and separate the lower waste acid layer; add p-dichlorobenzene to 1 times the weight ratio for purification and wash, and then separate the water layer; Neutralize to neutral with liquid caustic soda and discard the water layer. 153.1 g of 2-nitro-1,4-dichlorobenzene was obtained, which was directly used in the next reaction. The yield in this step was 97.7%.
[0032] Step 2: Preparation of 2-nitro-4-chloroaniline
[0033] Add 153.1g 2-nitro-1,4-dichlorobenzene obtained in the previous step, 2.2 times the ...
Embodiment 2
[0041] Step 1: Preparation of 2-nitro-1,4-dichlorobenzene
[0042] In a 500ml four-necked flask with a thermometer and a stirring device, start the stirring, add 120.0g of p-dichlorobenzene, 0.8 times the weight ratio of concentrated sulfuric acid, and add 1.04 times the molar amount of dichlorobenzene to nitric acid, and control the temperature of the drop. After the dripping is completed at 20~25℃, keep the reaction at 35~40℃ for 1 hour, GC will track the reaction to complete, and separate the lower waste acid layer; add p-dichlorobenzene to 1 times the weight ratio for purification and wash, and then separate the water layer; Neutralize to neutral with liquid caustic soda and discard the water layer. 153.4 g of 2-nitro-1,4-dichlorobenzene was obtained, which was directly used in the next reaction. The yield in this step was 97.9%.
[0043] Step 2: Preparation of 2-nitro-4-chloroaniline
[0044] Add 153.4g of 2-nitro-1,4-dichlorobenzene, 2.2 times the weight ratio of 2-nitro-1,4...
Embodiment 3
[0052] Step 1: Preparation of 2-nitro-1,4-dichlorobenzene
[0053] In a 1000 ml four-necked flask with a thermometer and a stirring device, turn on the stirring, add 240.0g p-dichlorobenzene, 0.8 times the weight ratio of concentrated sulfuric acid, dropwise add nitric acid 1.04 times the molar amount of p-dichlorobenzene, control the drop Adding temperature is 20~25℃, after dripping, keep incubating at 35~40℃ for 1 hour, GC will track the reaction to completion, and remove the lower waste acid layer; add p-dichlorobenzene to 1 times the weight ratio for purification and washing, and remove the water layer ; Then neutralize to neutral with liquid caustic soda, discard the water layer. 307.2 g of 2-nitro-1,4-dichlorobenzene was obtained, which was directly used in the next reaction. The yield in this step was 98.0%.
[0054] Step 2: Preparation of 2-nitro-4-chloroaniline
[0055] Add 307.2g 2-nitro-1,4-dichlorobenzene obtained in the previous step, 2.2 times the weight ratio of 2-n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com