Crystallization of deuteration Palbociclib isethionate
A technology of isethionate and crystallization, applied in the field of crystallization of pharmaceutical compounds, and can solve problems such as unreported crystallization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0046]
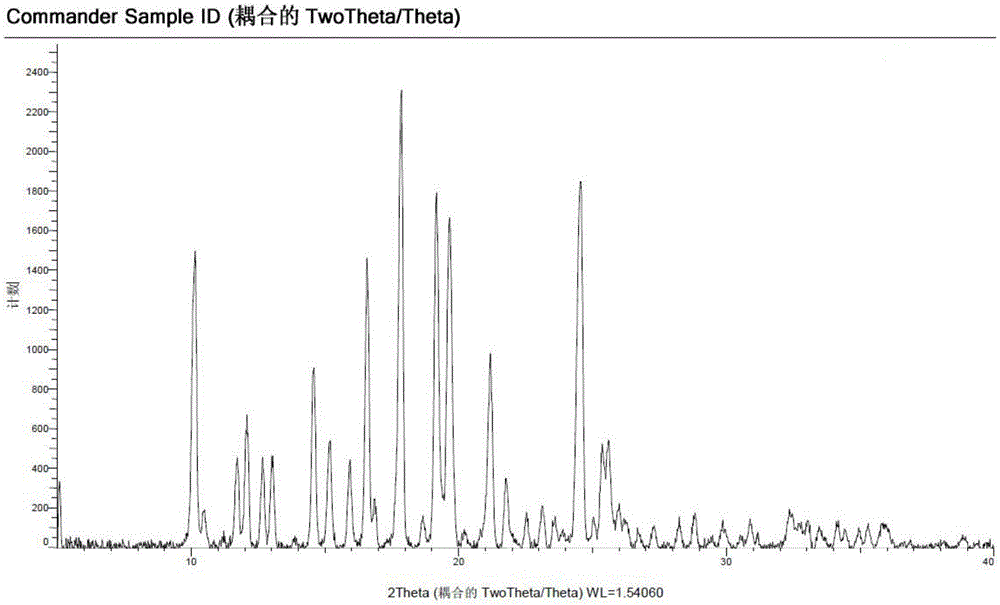
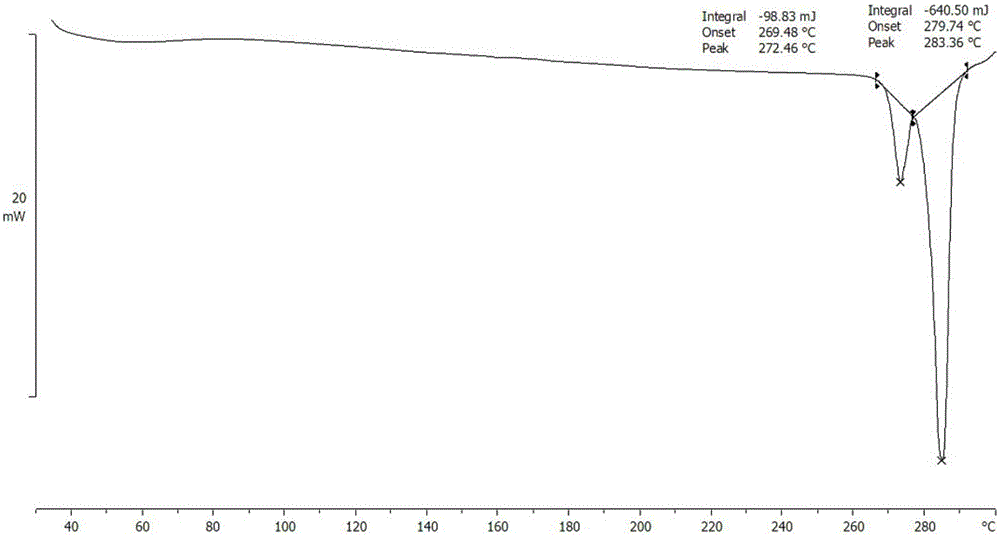
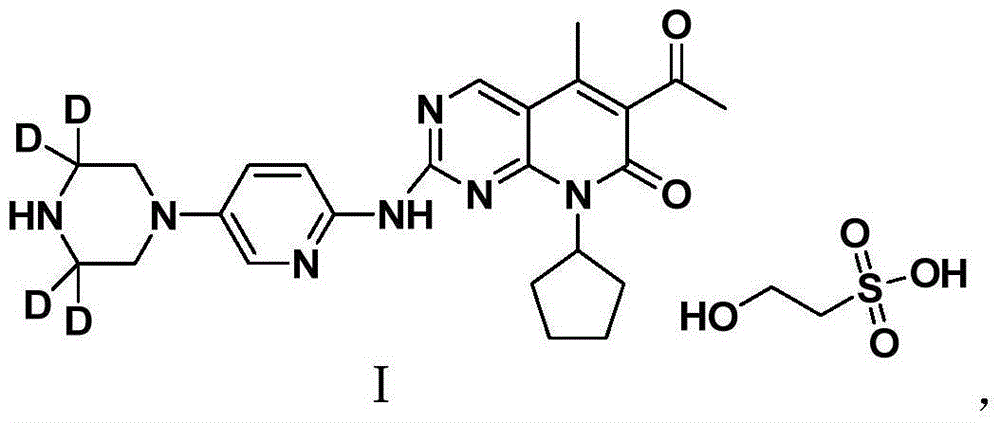
[0047] Add compound II (0.5 g, 1.11 mmol) and methanol (2 mL) to the reaction flask respectively, and stir at room temperature to form a suspension. Isethionic acid (0.14 g, 1.11 mmol) was added dropwise to the suspension. The solid dissolved rapidly and then precipitated a yellow solid. Methanol (2 mL) was added, and the temperature was raised to 45° C. and stirred for 4 hours. Slowly let cool to room temperature, and cooled and precipitated at 4°C overnight. A yellow solid was obtained by suction filtration, and dried in a vacuum oven at 50°C to obtain deuterated palbociclib isethionate crystals (0.55 g) as shown in Formula I.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com