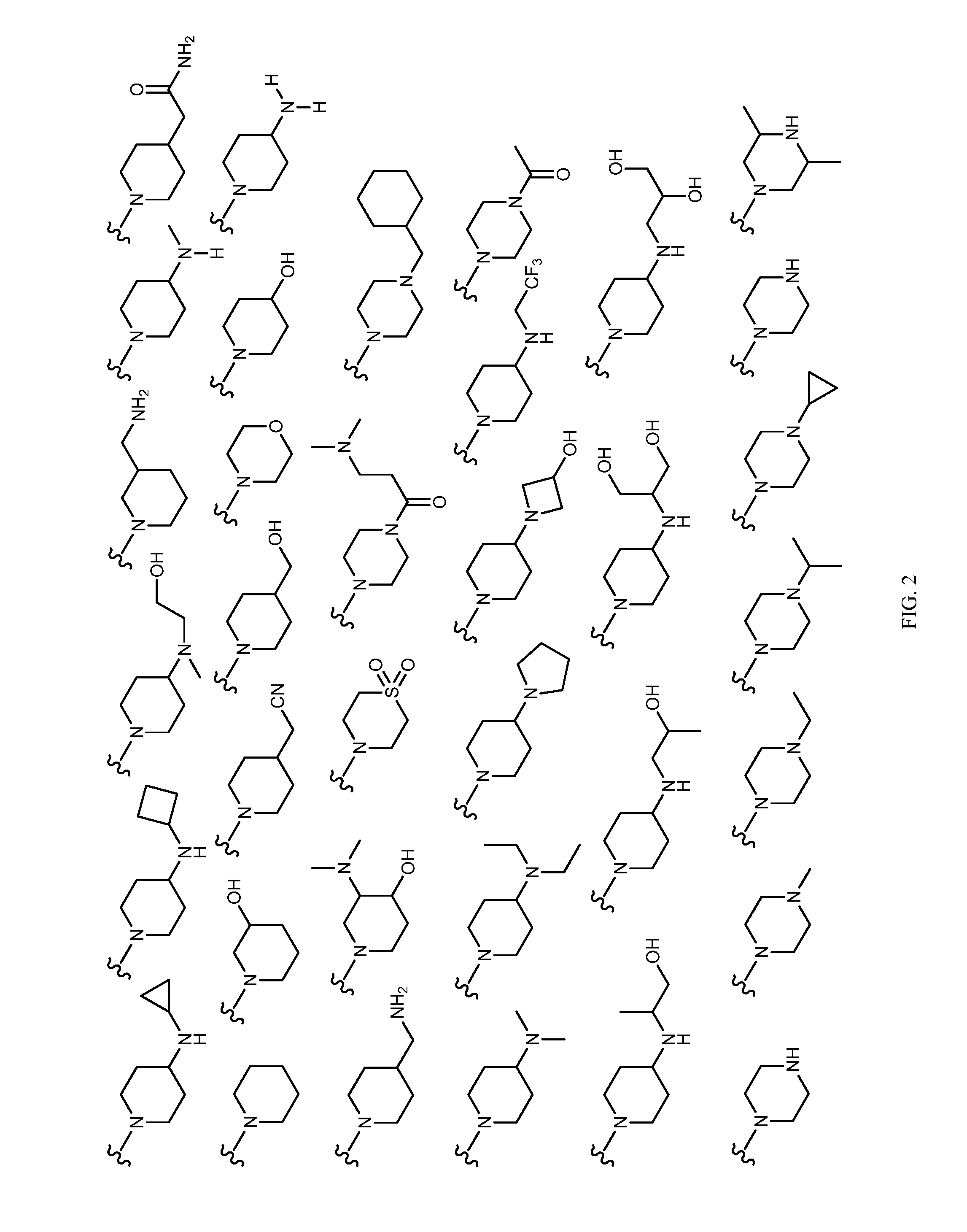
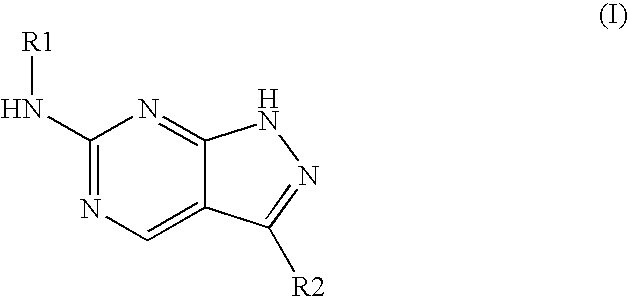
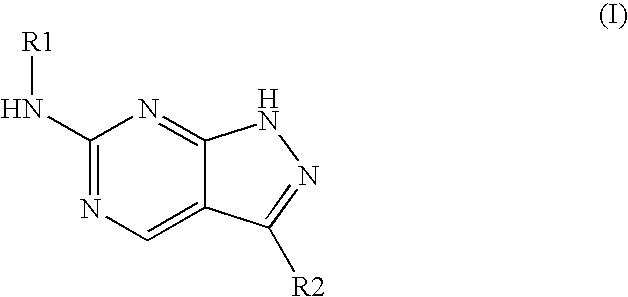
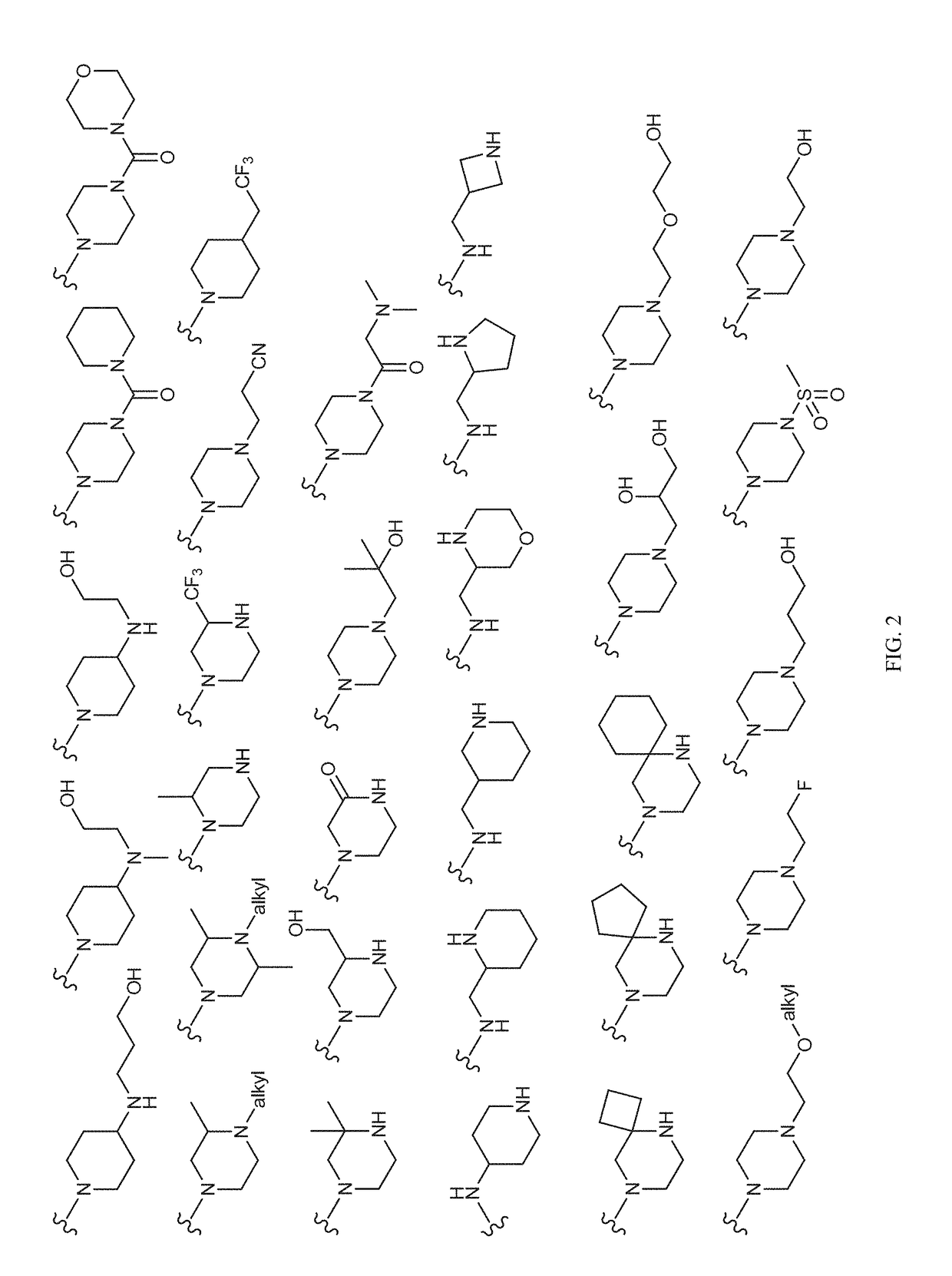
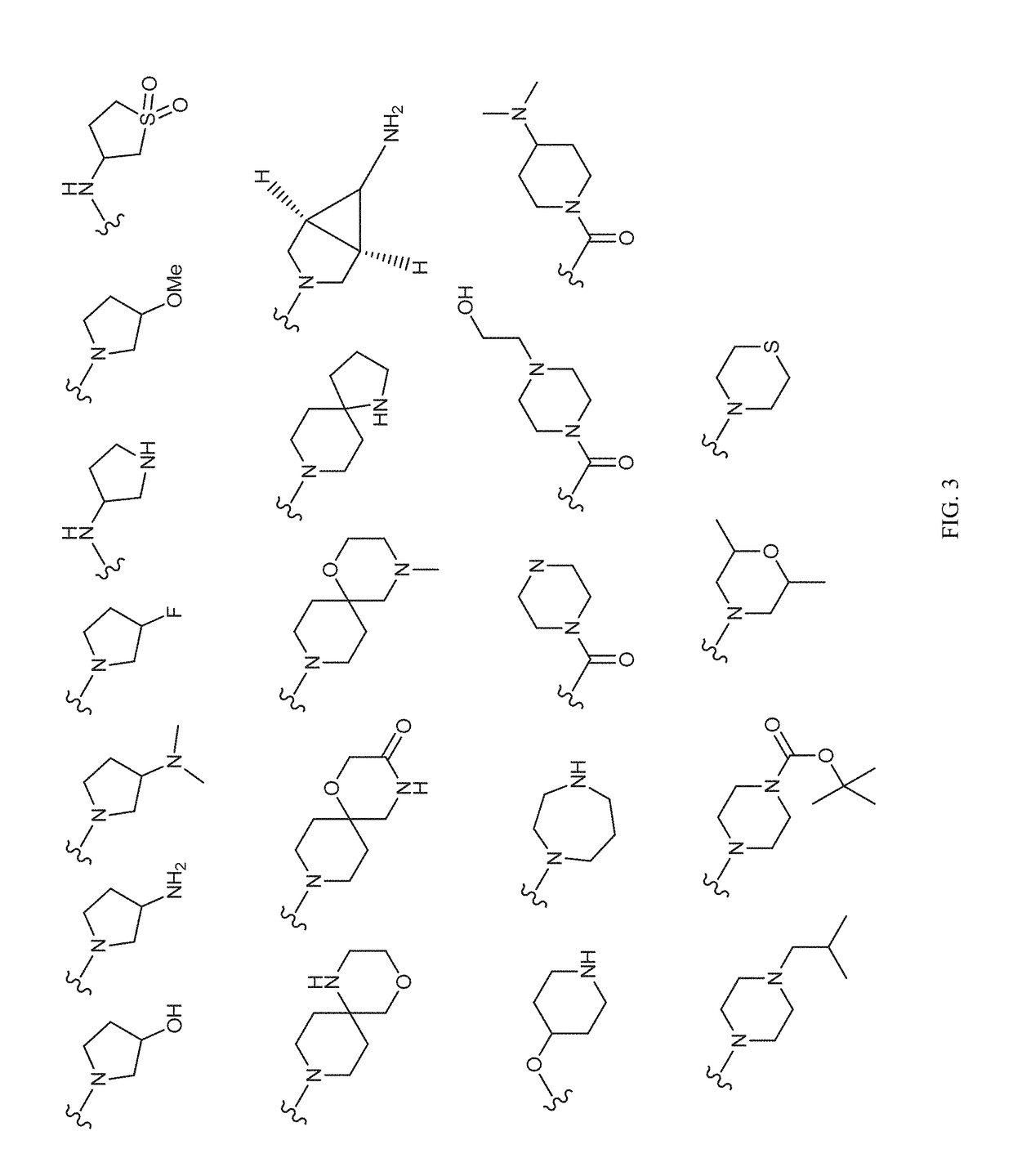
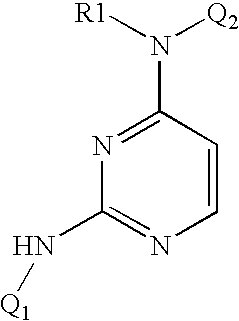
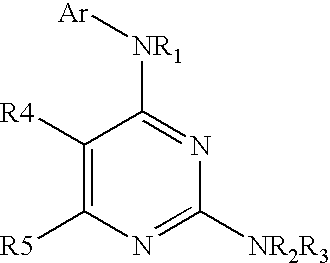
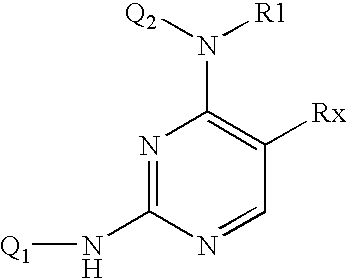
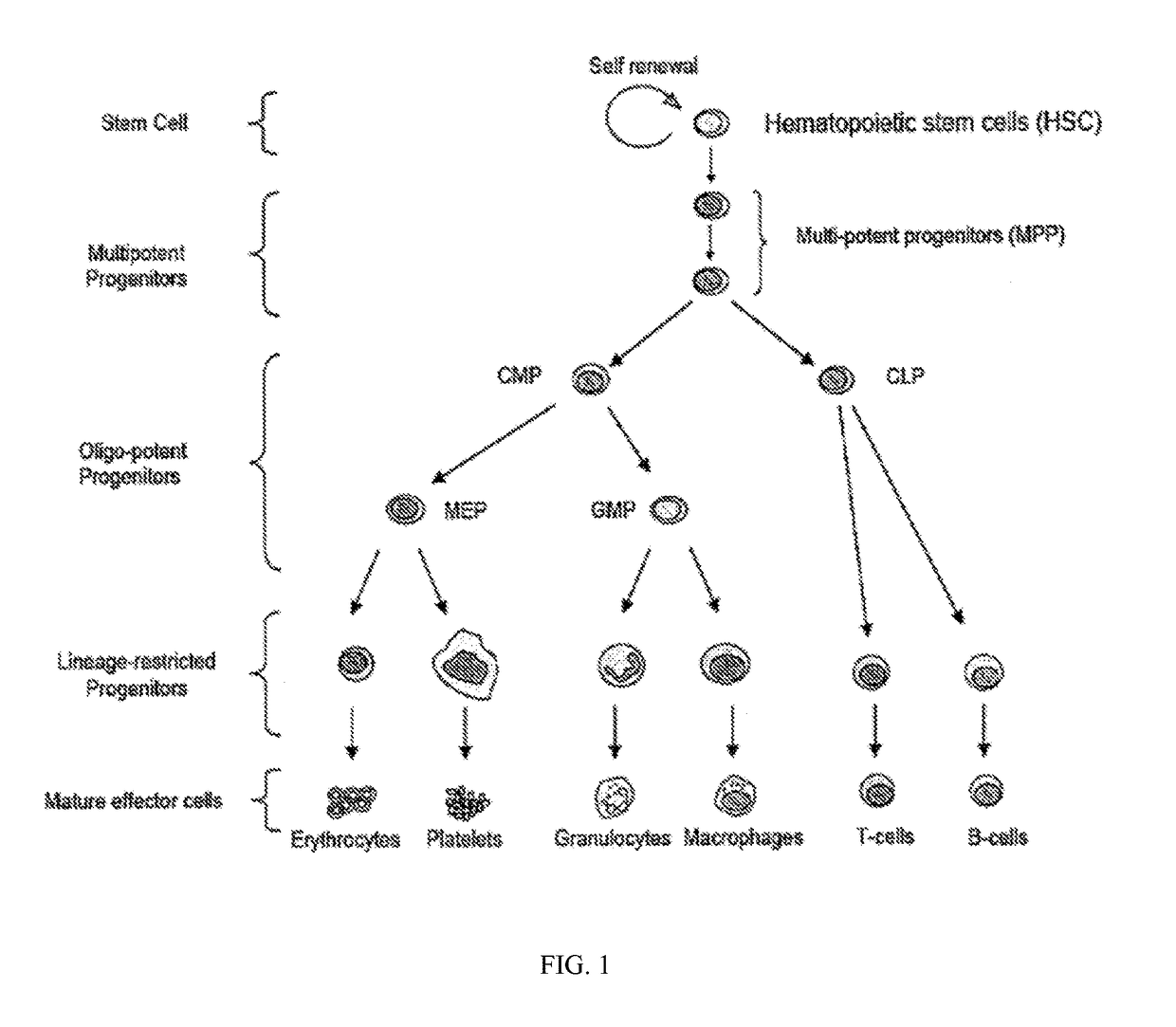
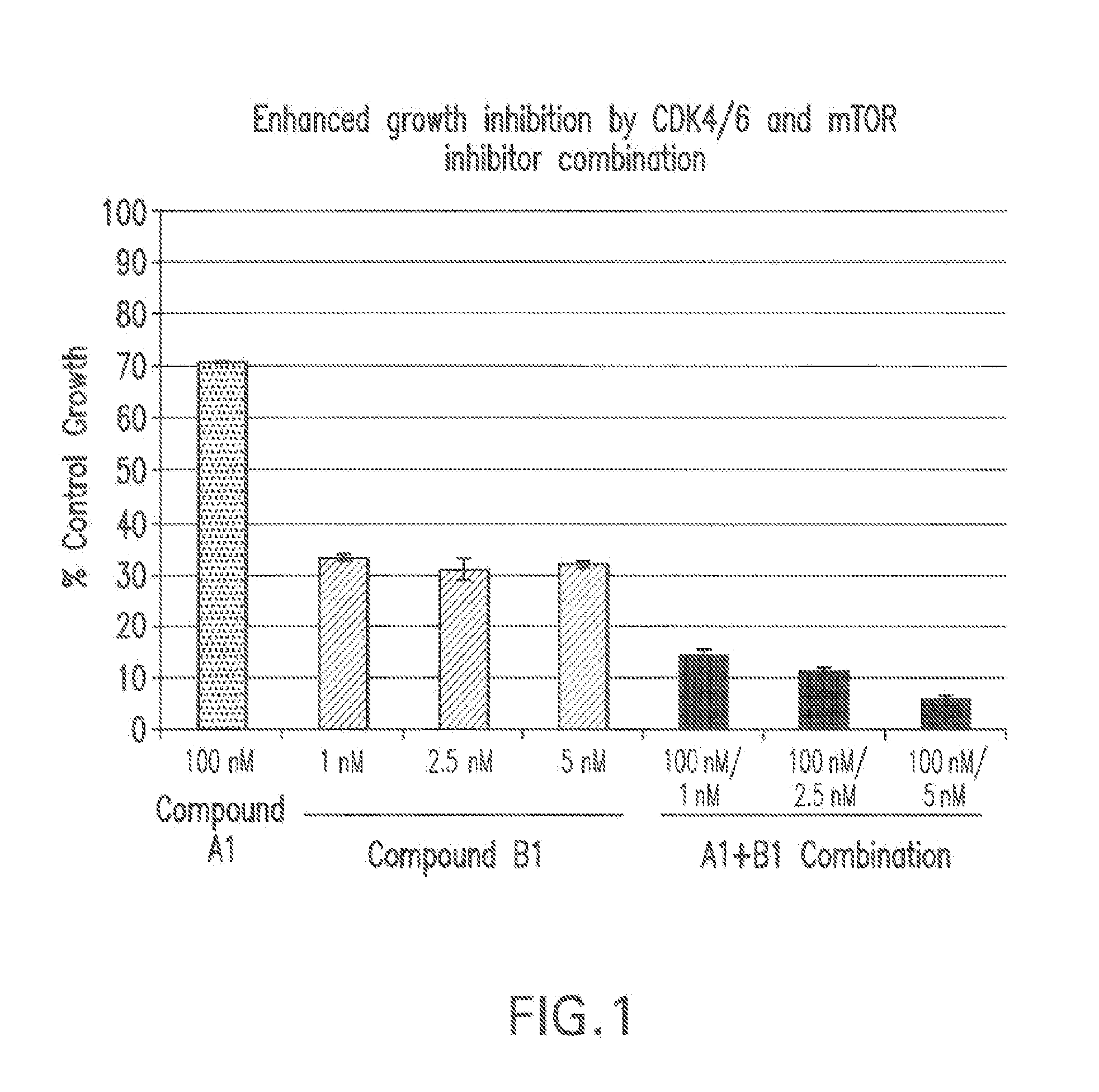
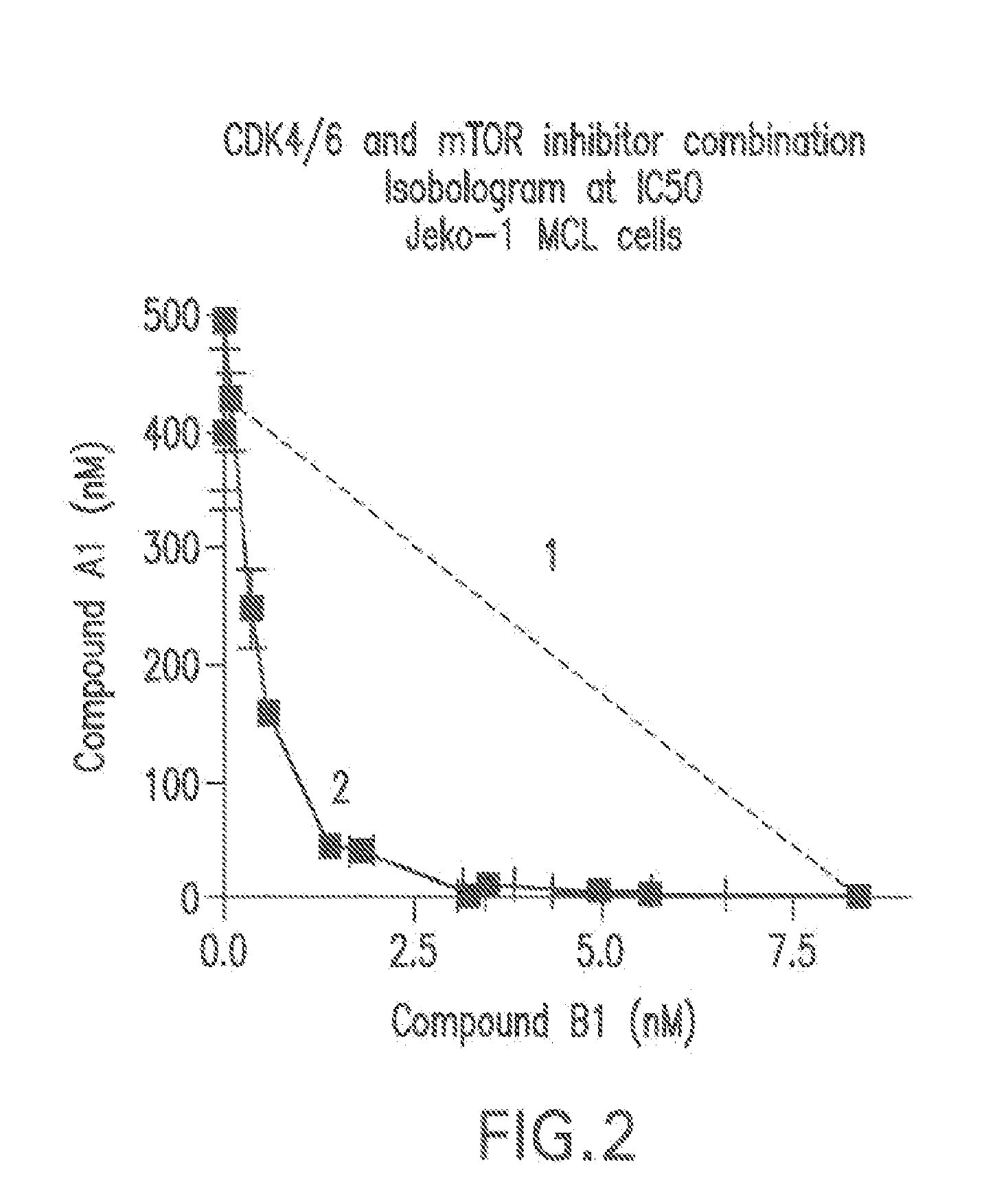
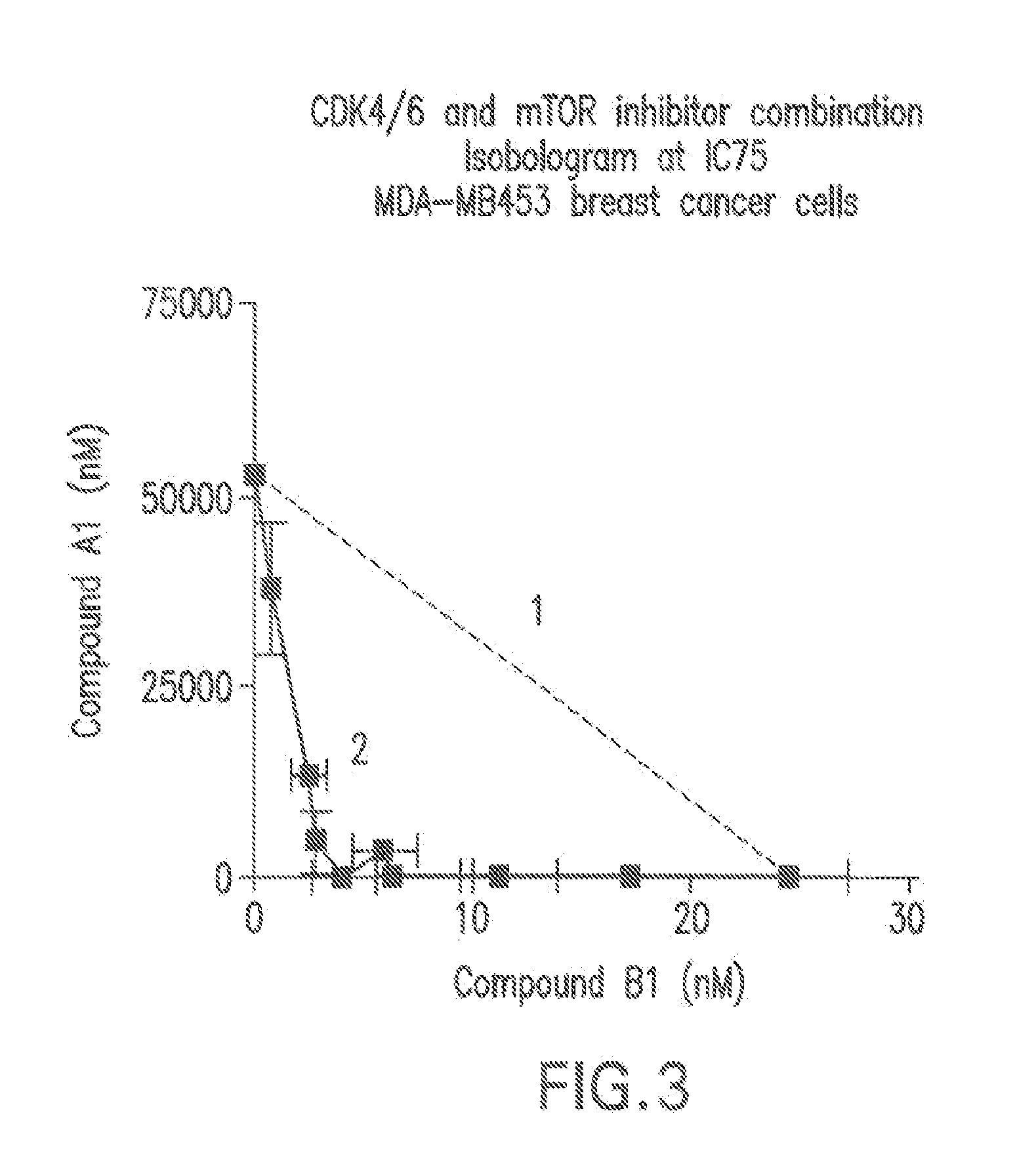
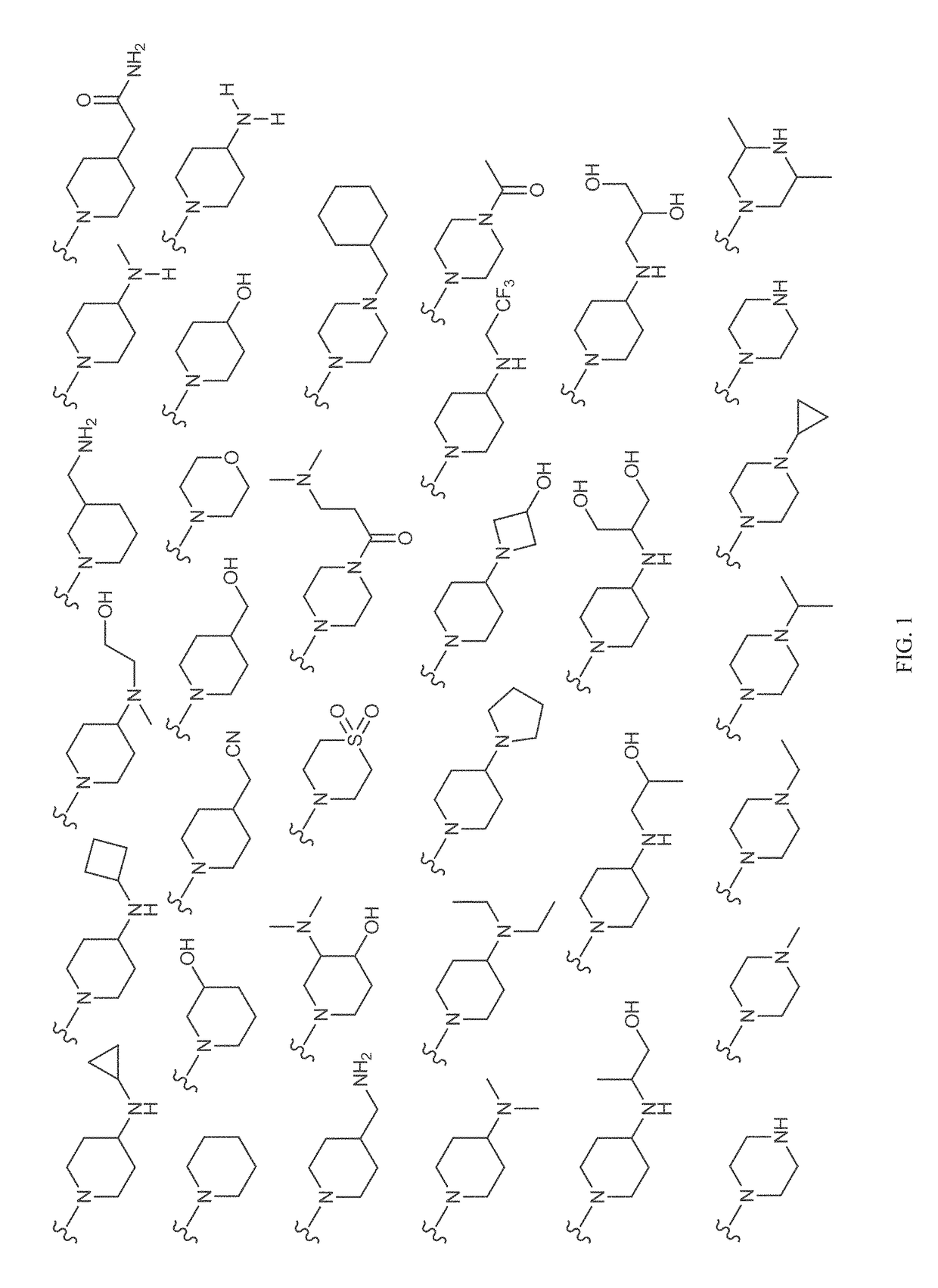
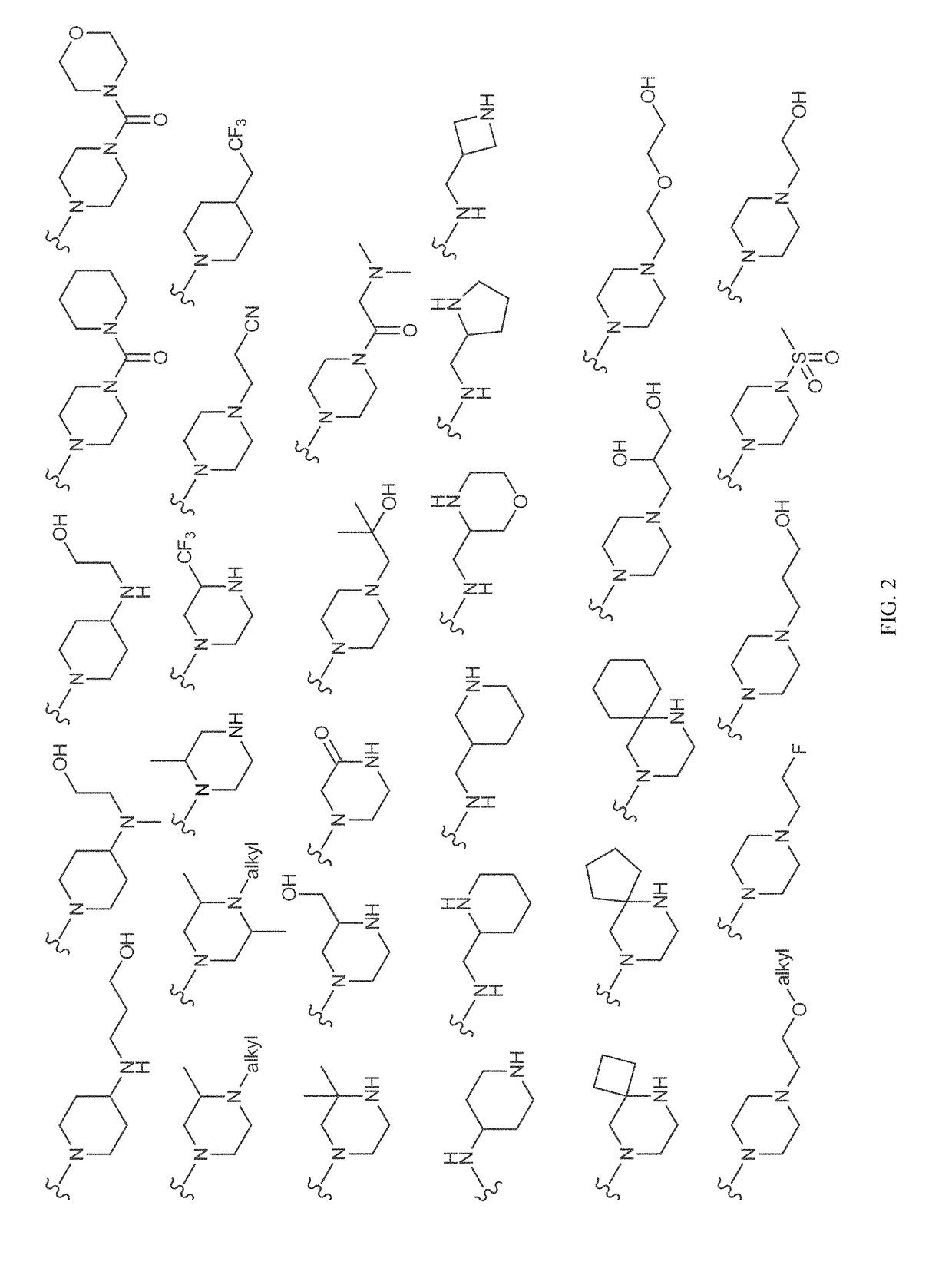
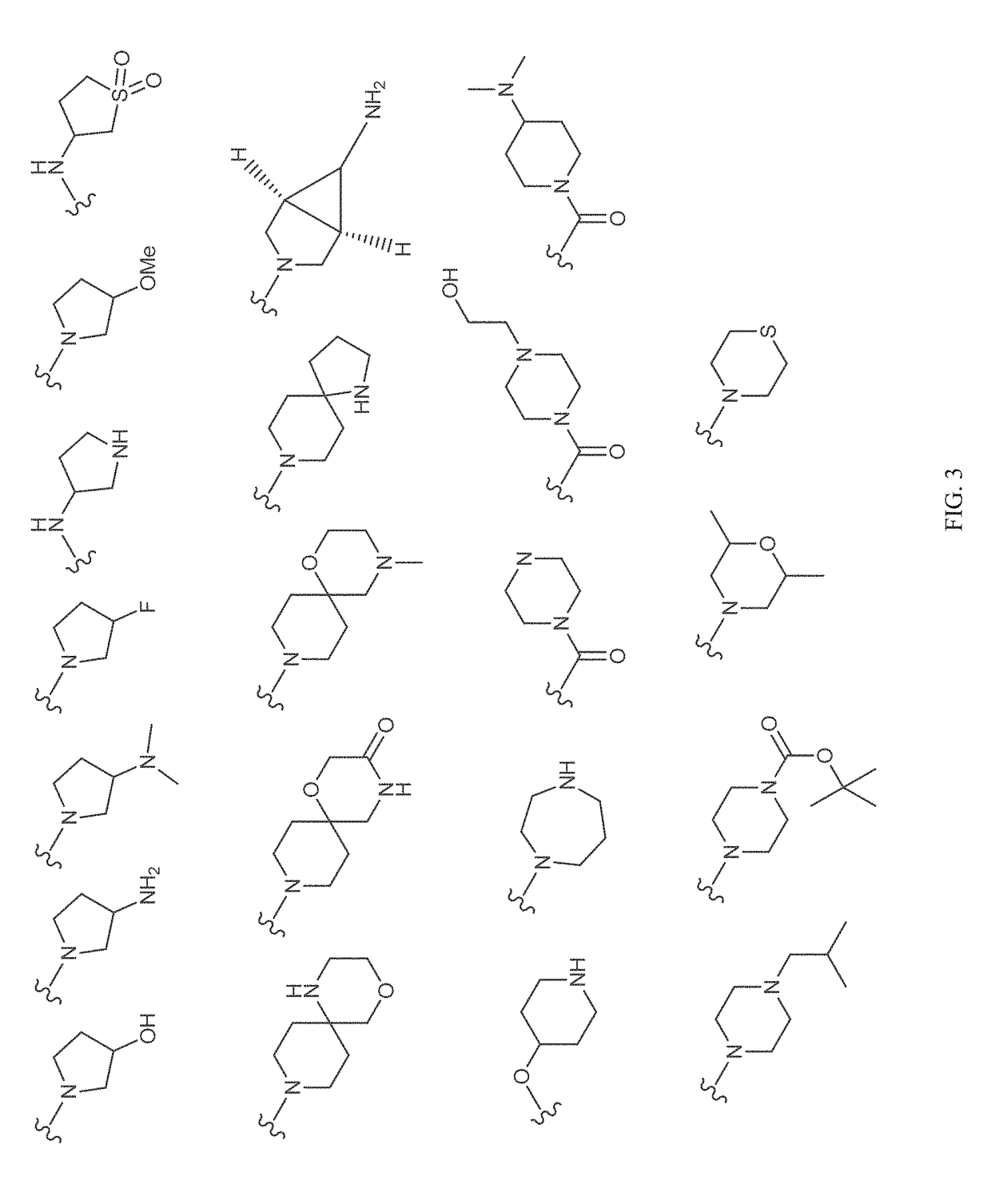
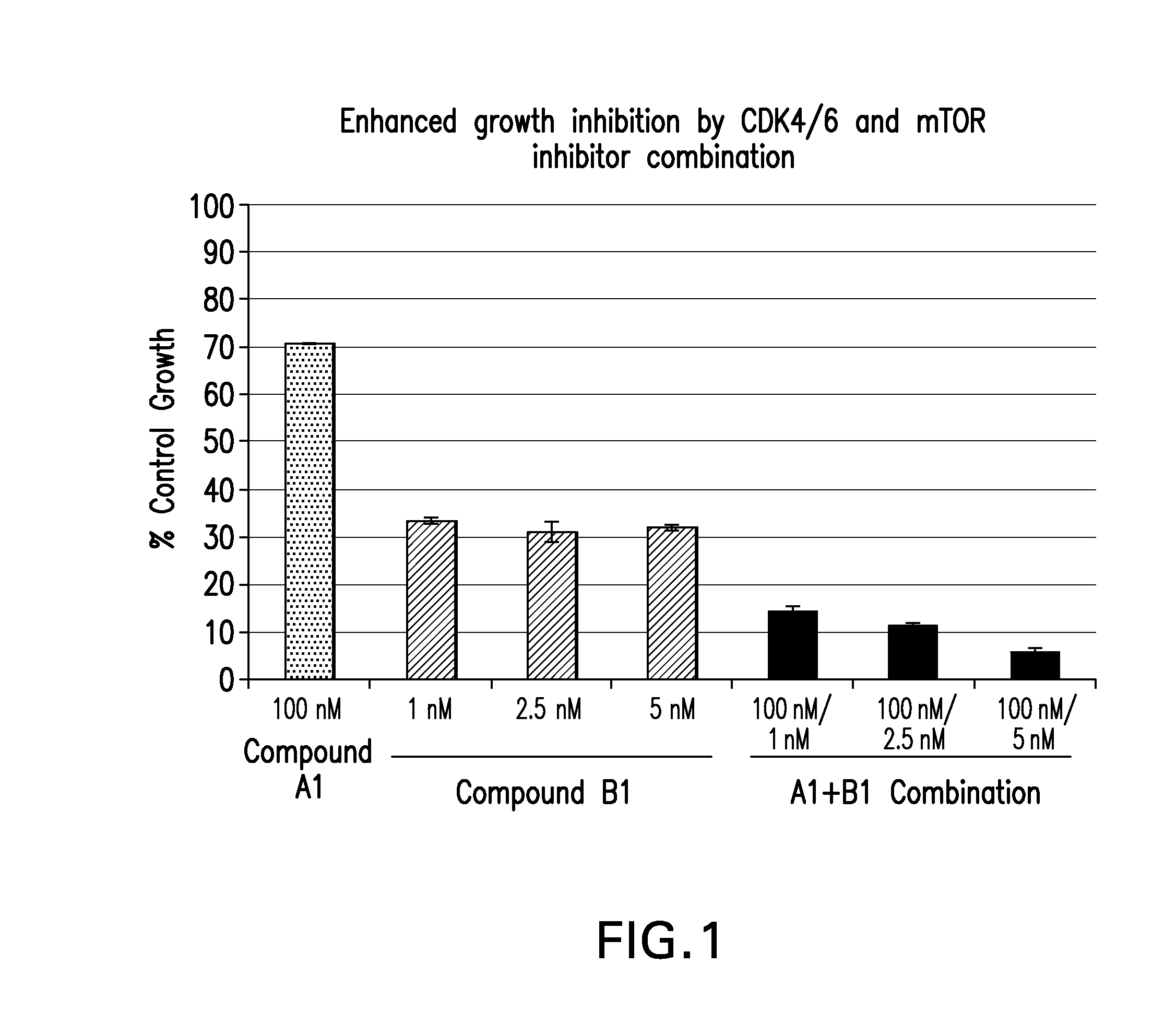
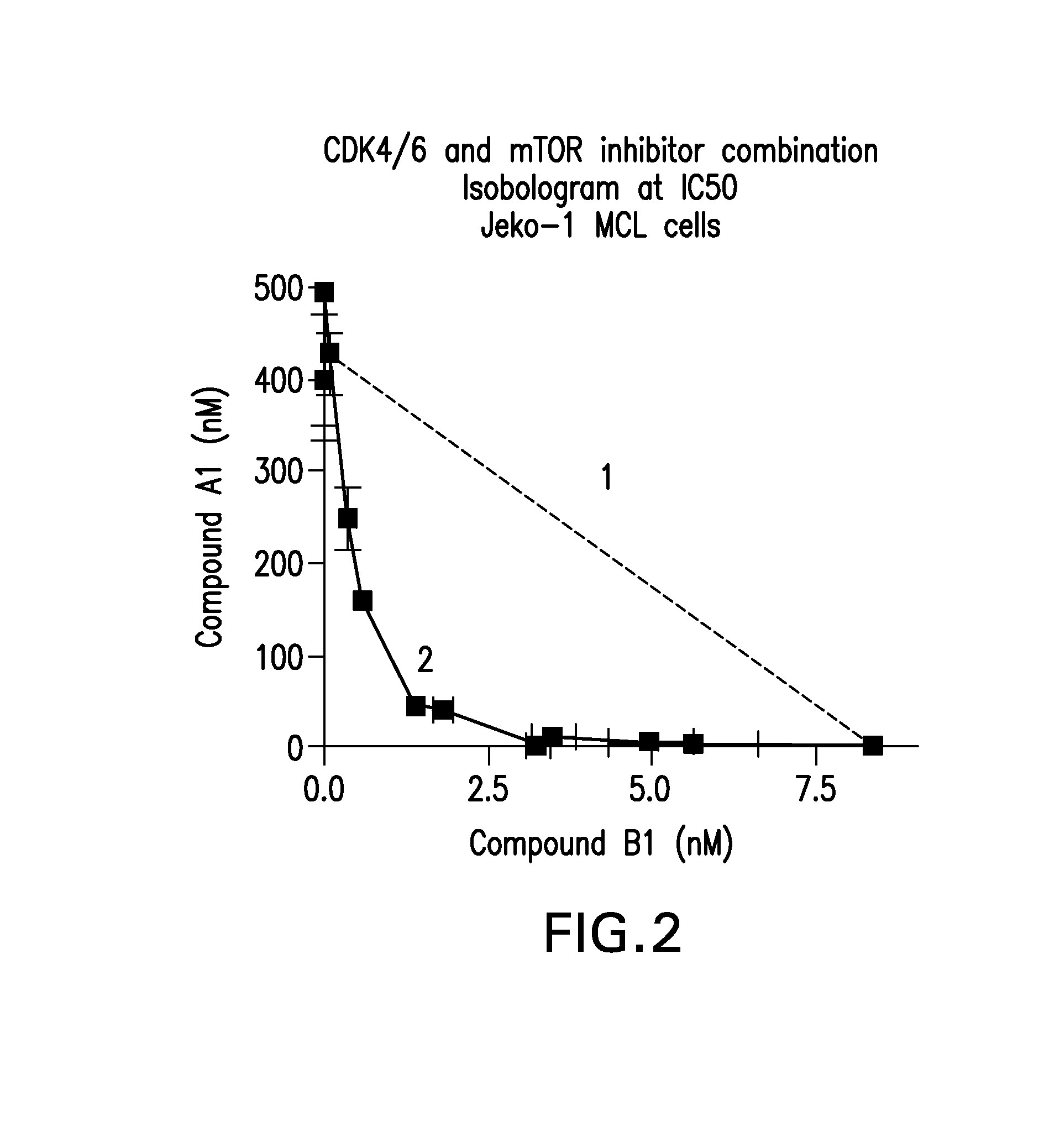
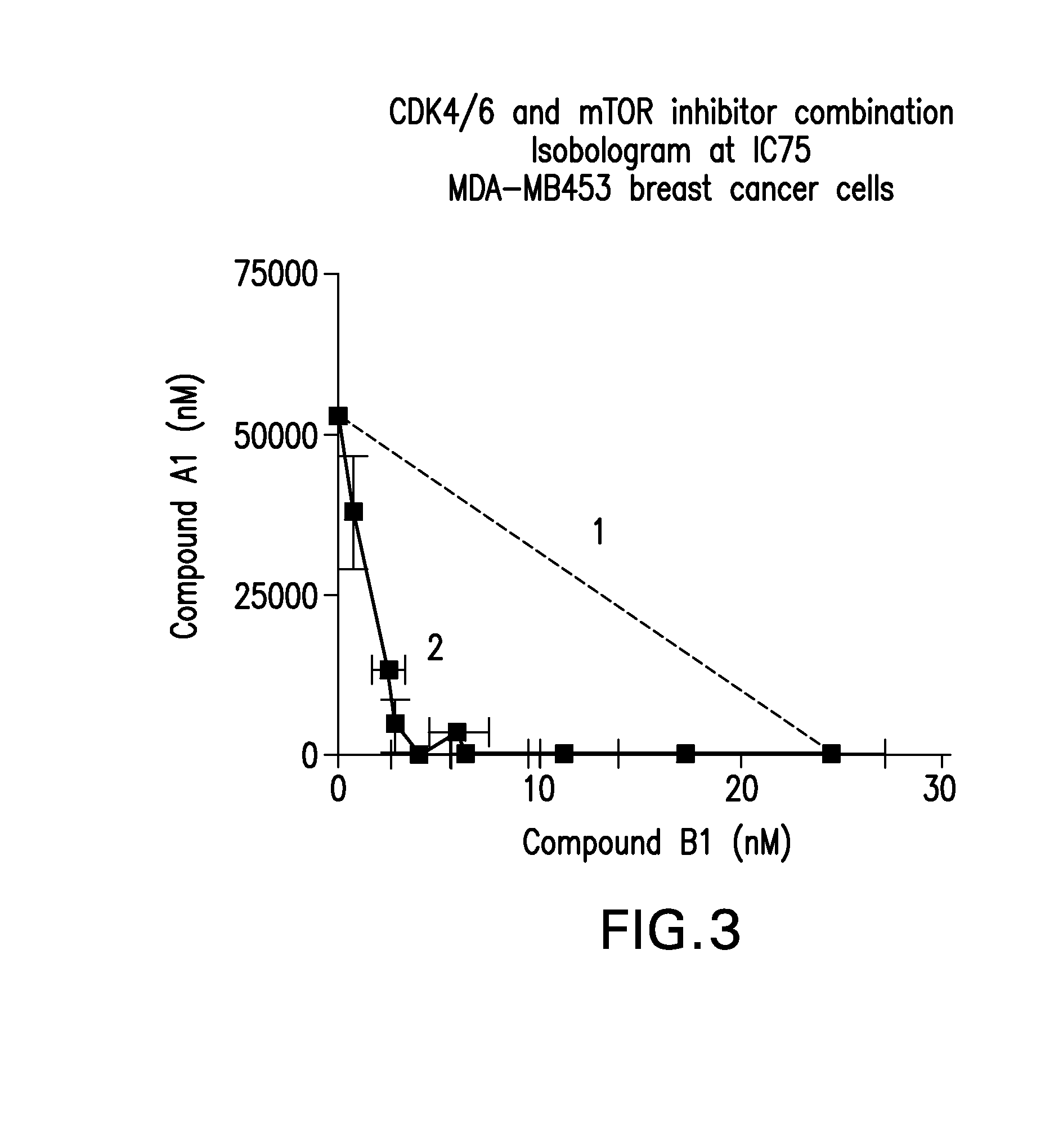
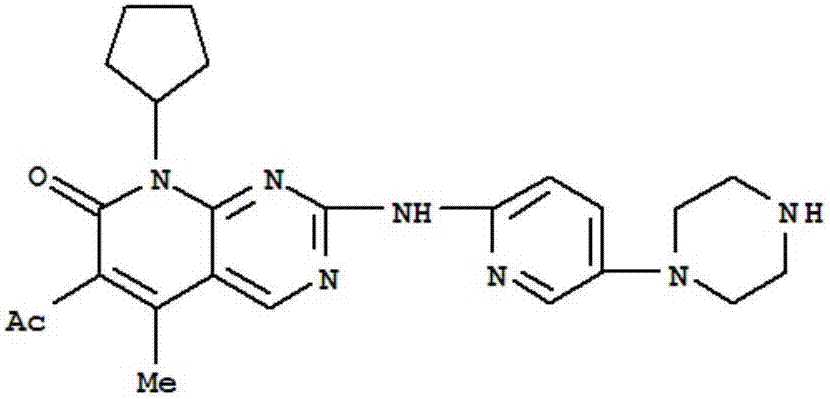
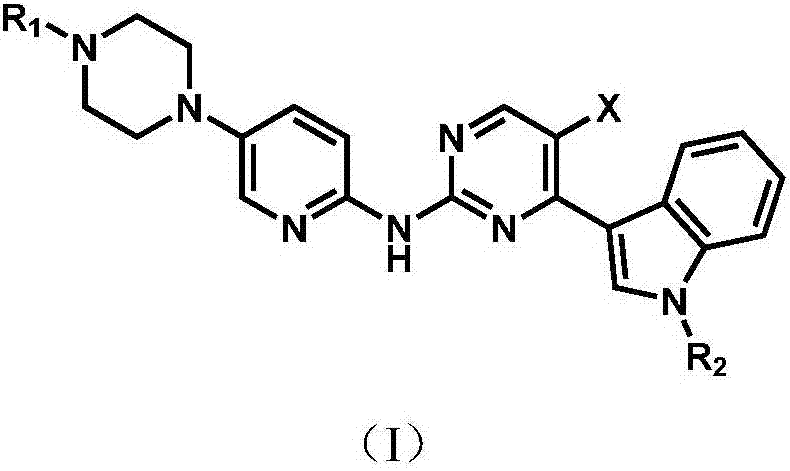
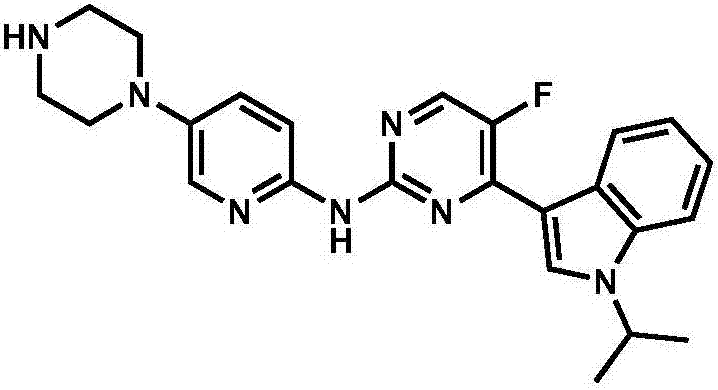
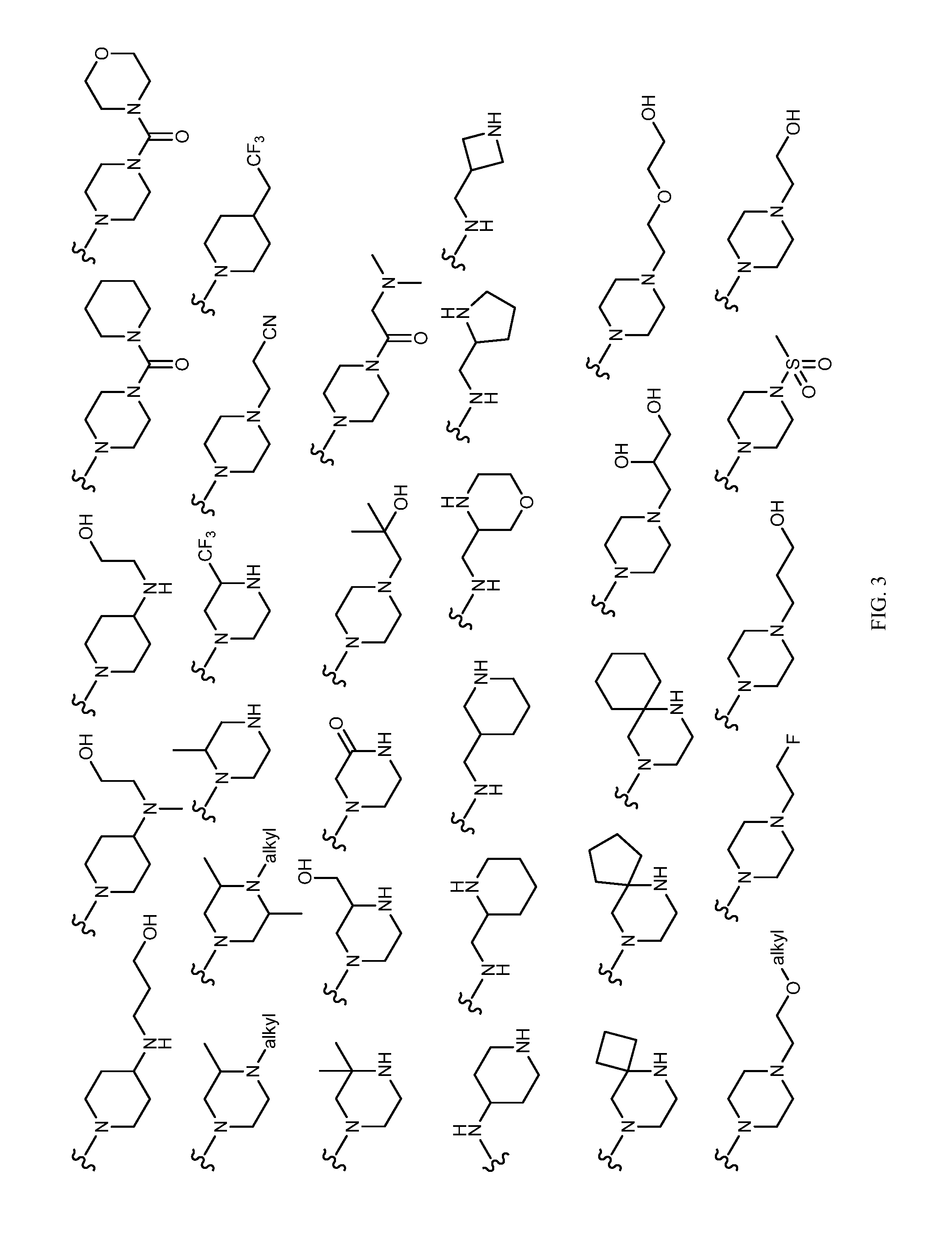Patents
Literature
38 results about "Cyclin-dependent kinase 4" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cyclin-dependent kinase 4 also known as cell division protein kinase 4 is an enzyme that in humans is encoded by the CDK4 gene. CDK4 is a member of the cyclin-dependent kinase family.
Combinations of signal transduction inhibitors
InactiveUS20050222163A1Promote growthPromote proliferationOrganic active ingredientsOrganic chemistryAnticarcinogenCell Cycle Inhibition
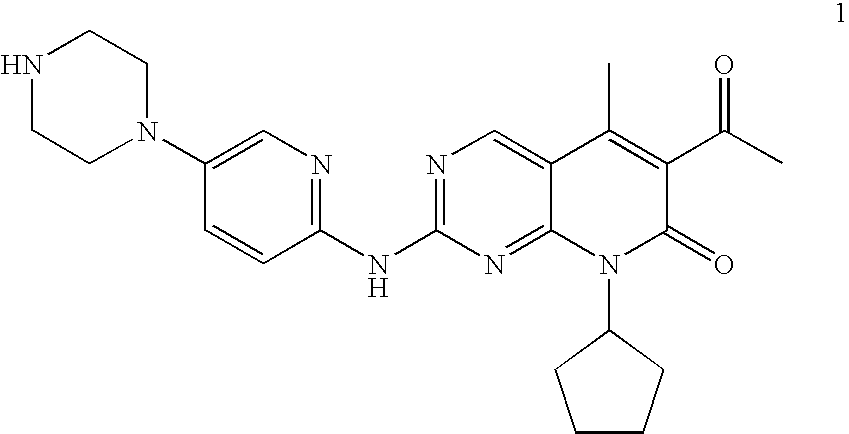
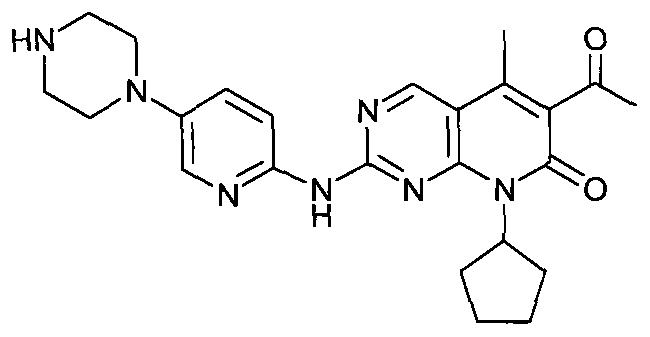
The present invention relates to methods for treating cancer comprising utilizing a combination of signal transduction inhibitors. More specifically, the present invention relates to combinations of so called cell cycle inhibitors with mitogen stimulated kinase signal transduction inhibitors, more specifically combinations of CDK inhibitors with mitogen stimulated kinase signal transduction inhibitors, more preferably MEK inhibitors. Other embodiments of the invention relate to additional combinations of the aforesaid combinations with standard anti-cancer agents such as cytotoxic agents, palliatives and antiangiogenics. Most specifically this invention relates to combinations of 6-acetyl-8-cyclopentyl-5-methyl-2-(5-piperazin-1-yl-pyridin-2-ylamino)-8H-pyrido[2,3-d]pyrimidin-7-one including salt forms, which is a selective cyclin-dependent kinase 4 (CDK4) inhibitor, in combination with one or more MEK inhibitors, most preferably N-[(R)-2,3-dihydroxy-propoxy]-3,4-difluoro-2-(2-fluoro-4-iodo-phenylamino)-benzamide. The aforementioned combinations are useful for treating inflammation and cell proliferative diseases such as cancer and restenosis.
Owner:PFIZER INC
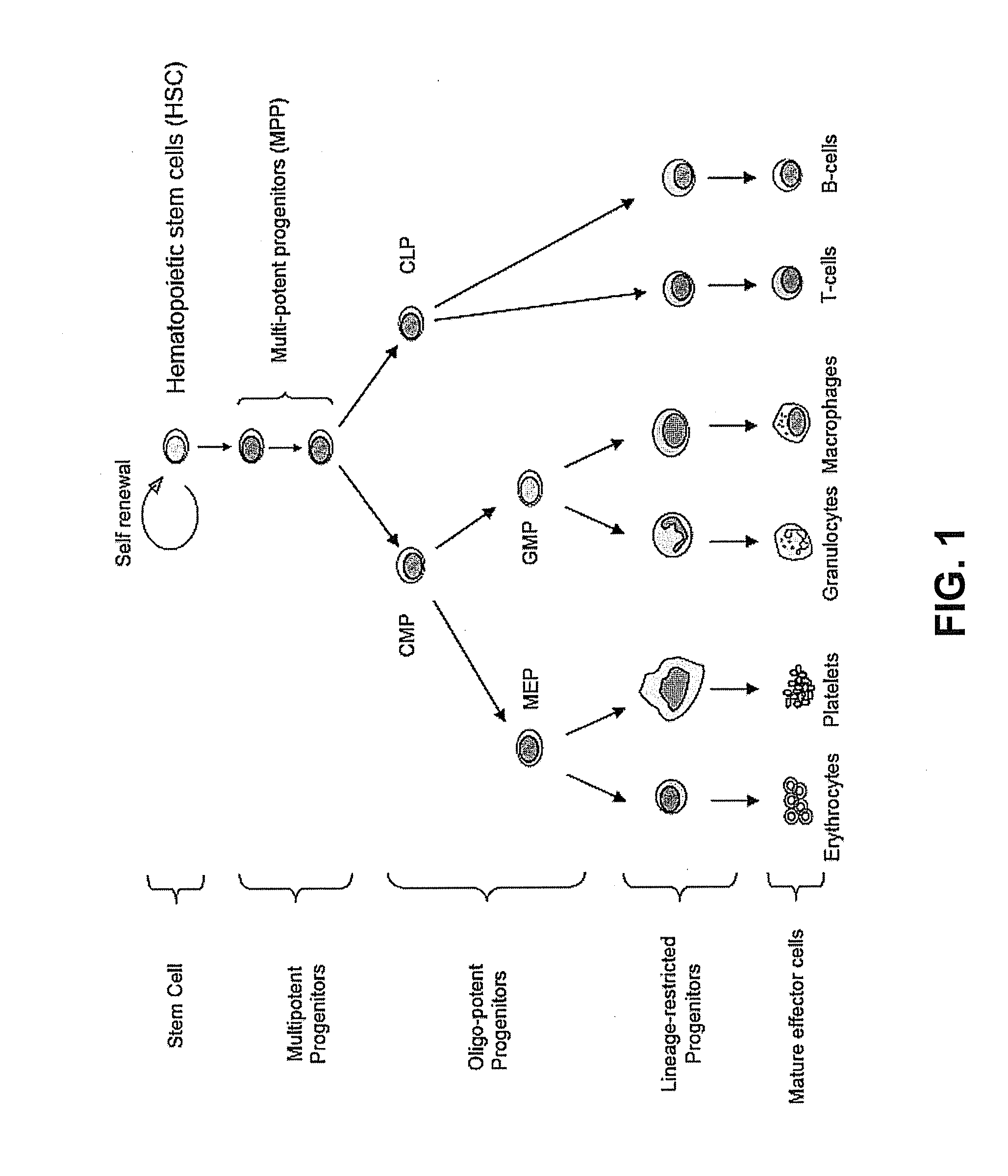
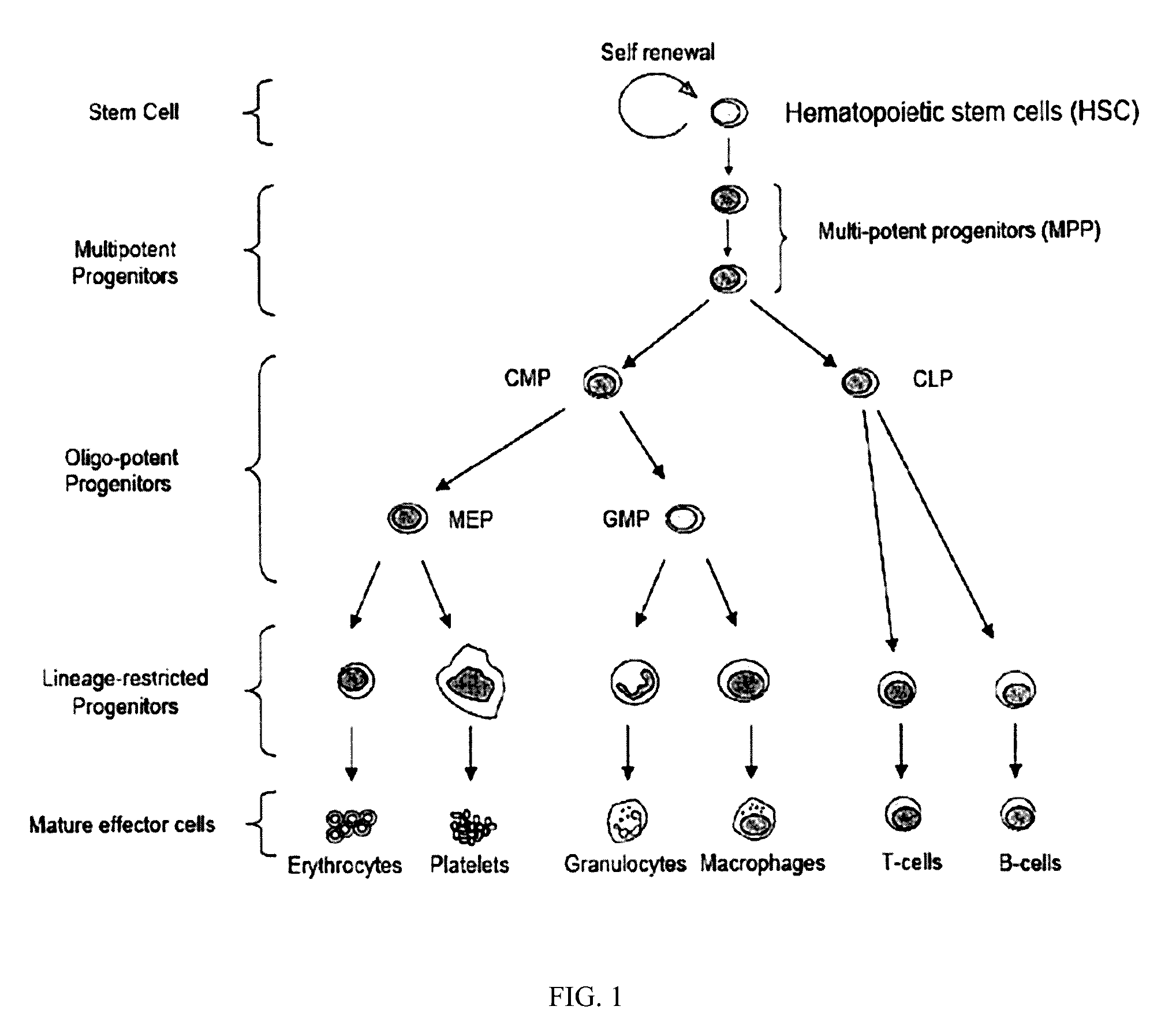
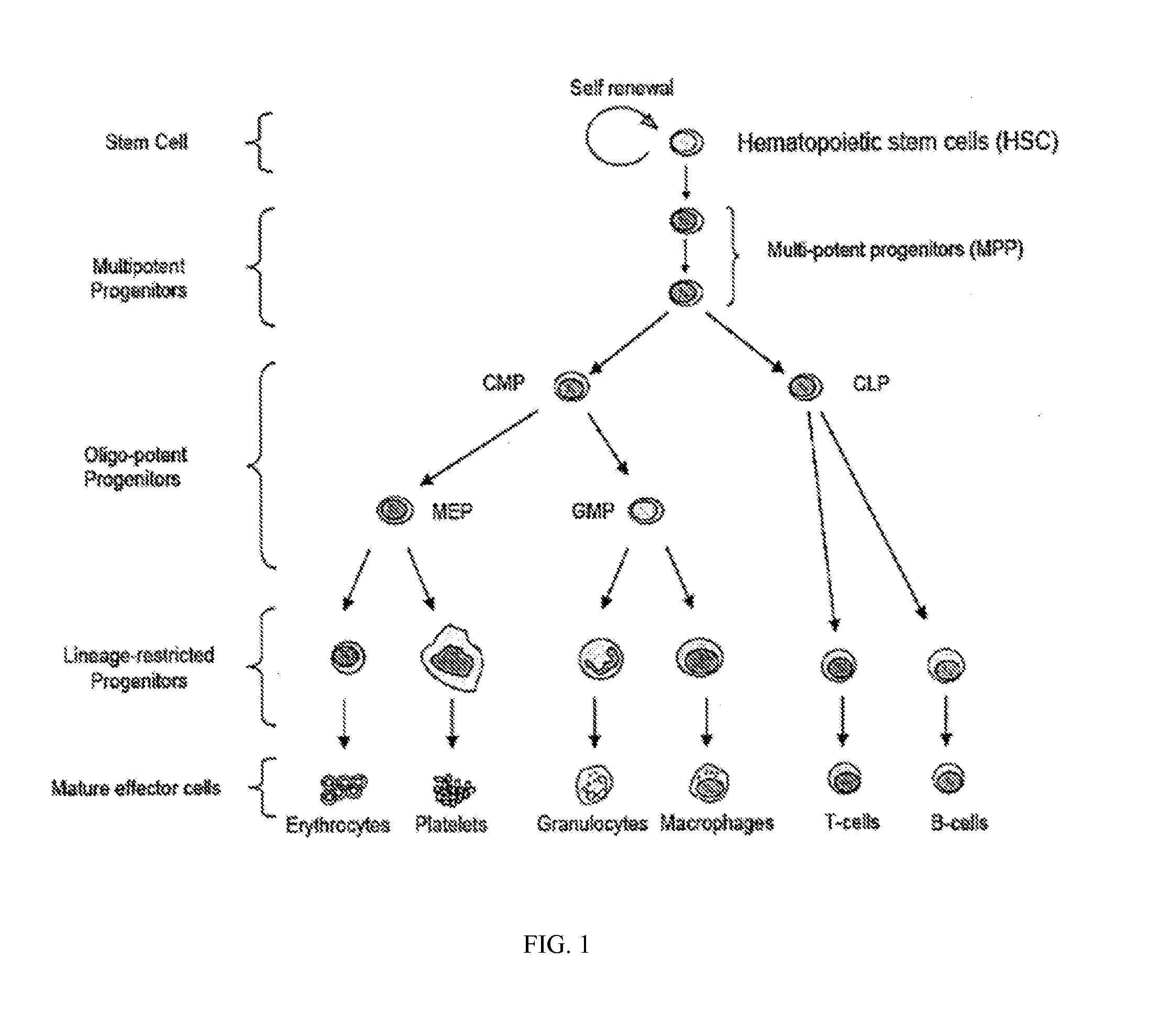
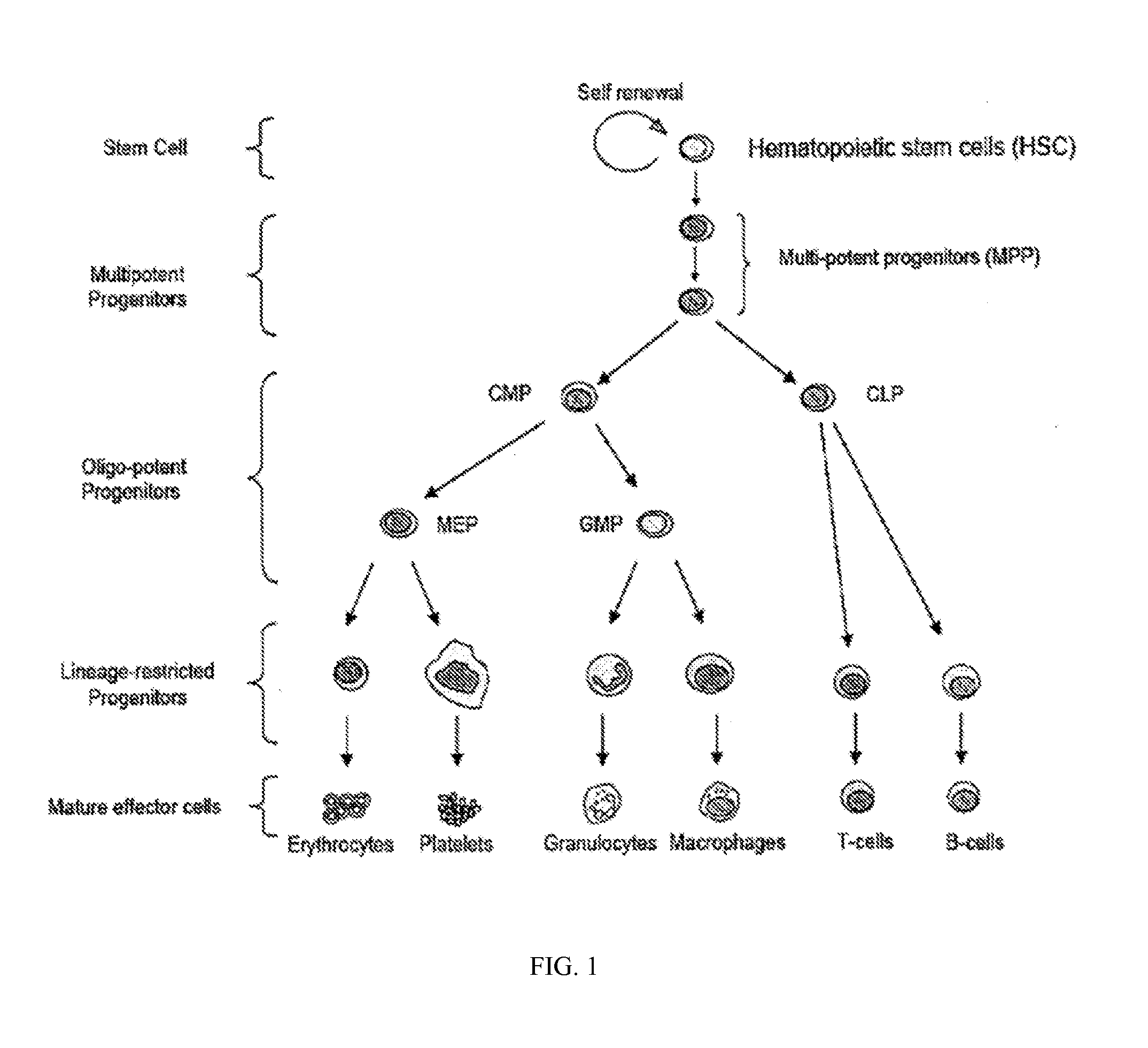
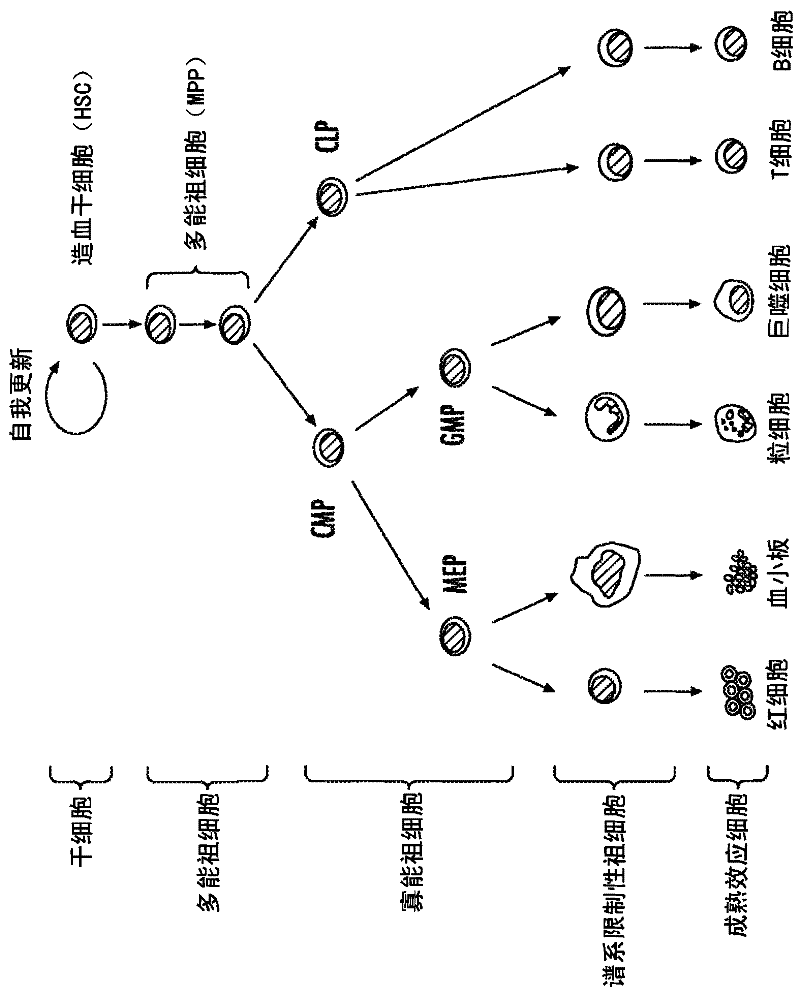
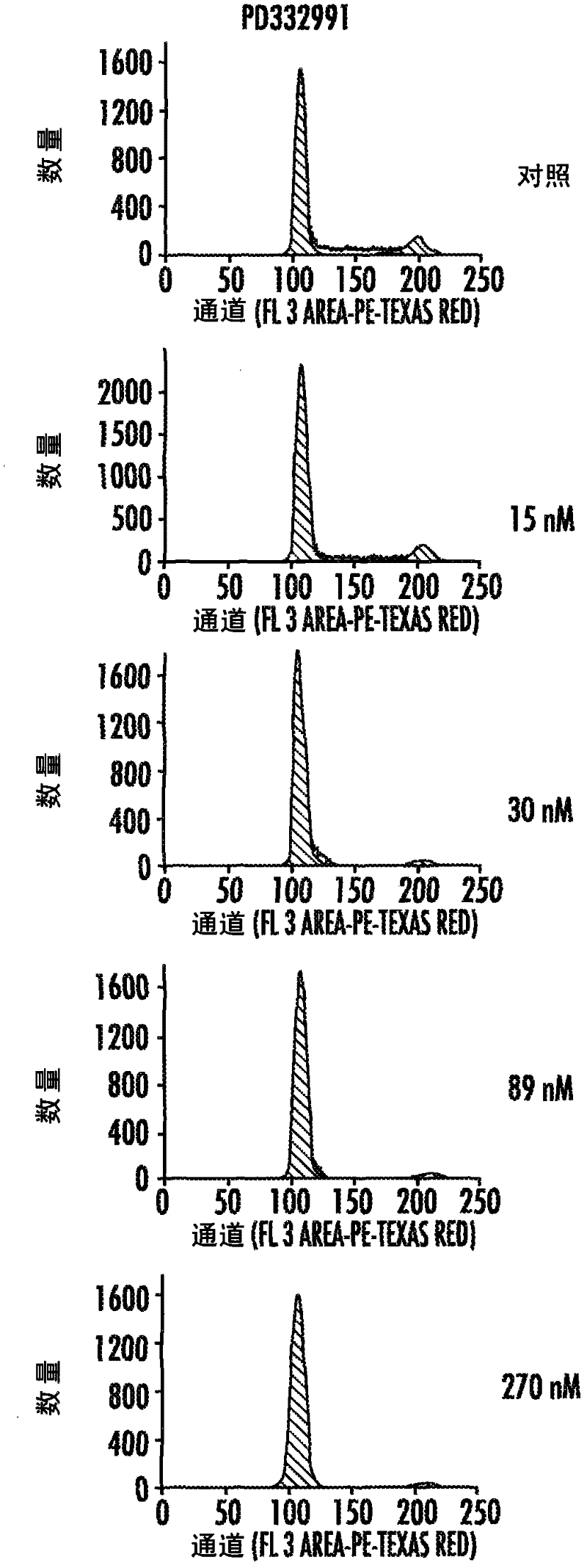
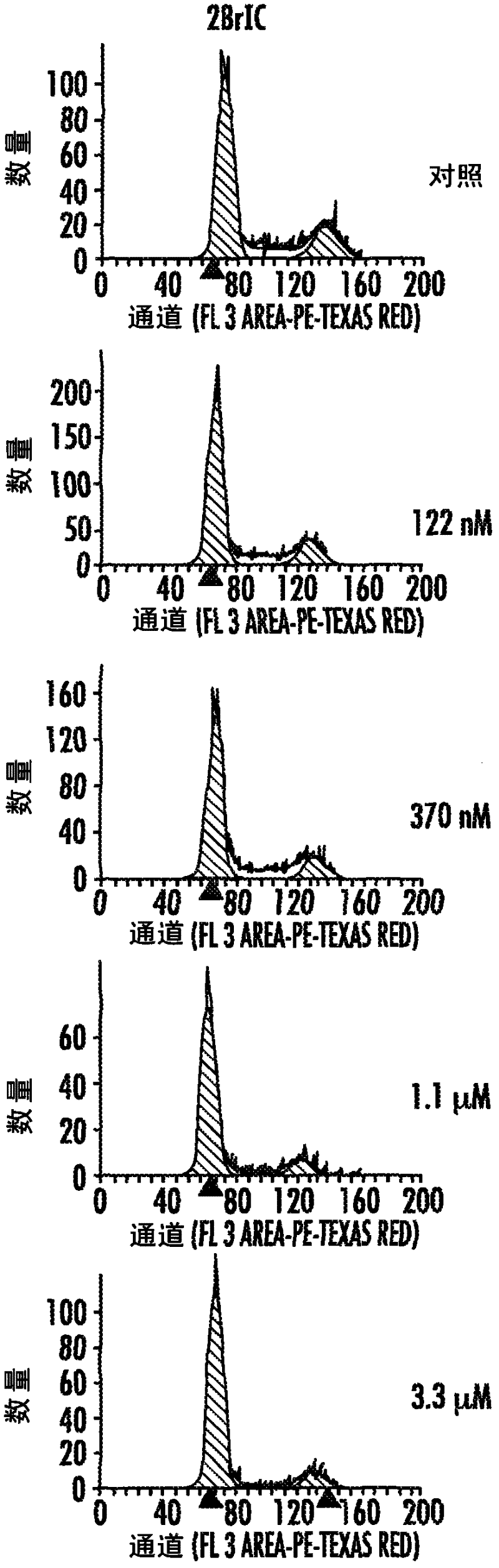
HSPC-Sparing Treatments for RB-Positive Abnormal Cellular Proliferation
ActiveUS20140271466A1MinimizingDeleterious effectBiocideOrganic chemistryProgenitorTreatment choices
This invention is in the area of improved compounds for and methods of treating selected RB-positive cancers and other Rb-positive abnormal cellular proliferative disorders while minimizing the deleterious effects on healthy cells, for example healthy Hematopoietic Stem Cells and Progenitor Cells (HSPCs), associated with current treatment modalities. In one aspect, improved treatment of select RB-positive cancers is disclosed using specific compounds disclosed herein. In certain embodiments, the compounds described herein act as highly selective and, in certain embodiments, short, transiently-acting cyclin-dependent kinase 4 / 6 (CDK 4 / 6) inhibitors when administered to subjects.
Owner:G1 THERAPEUTICS INC
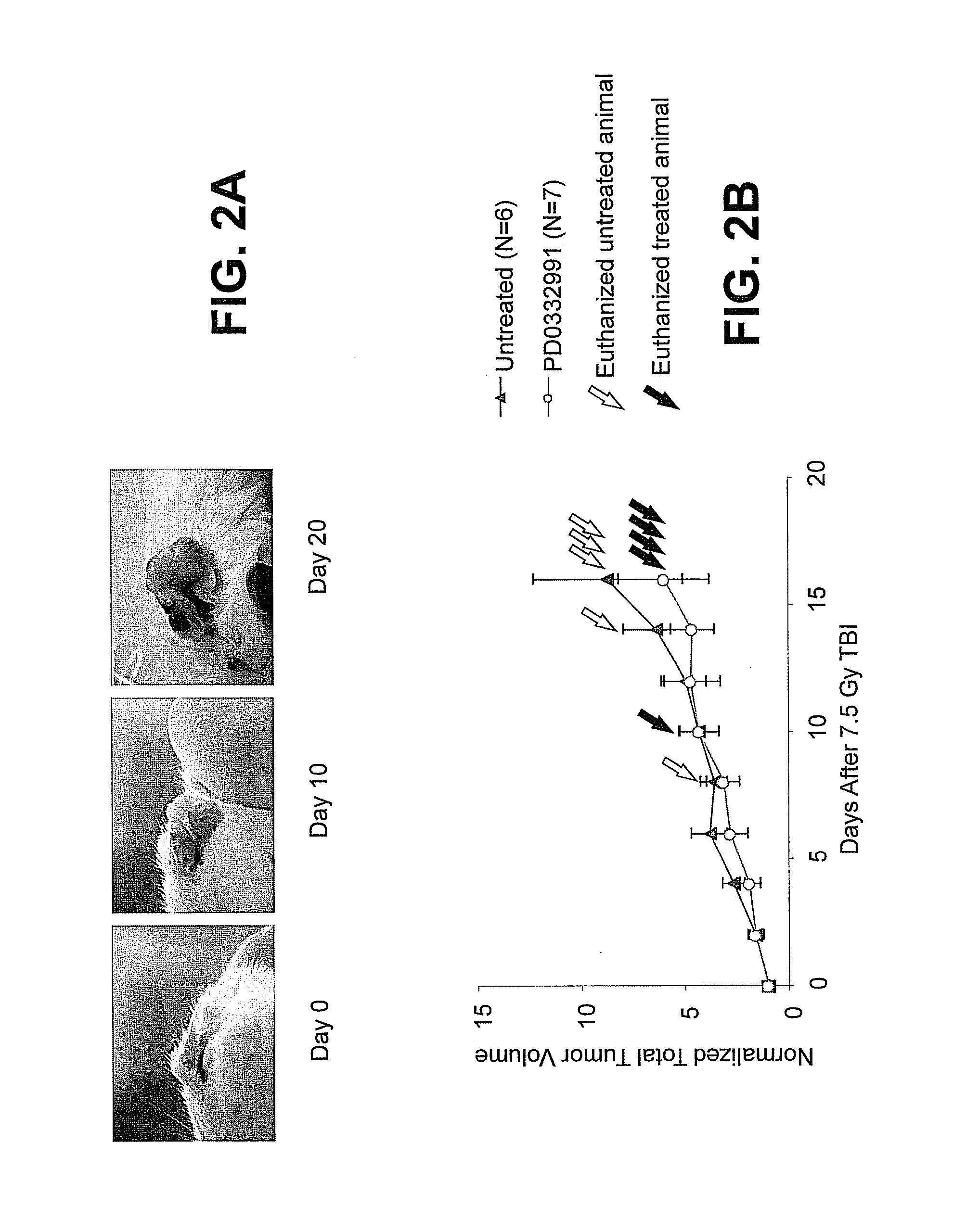
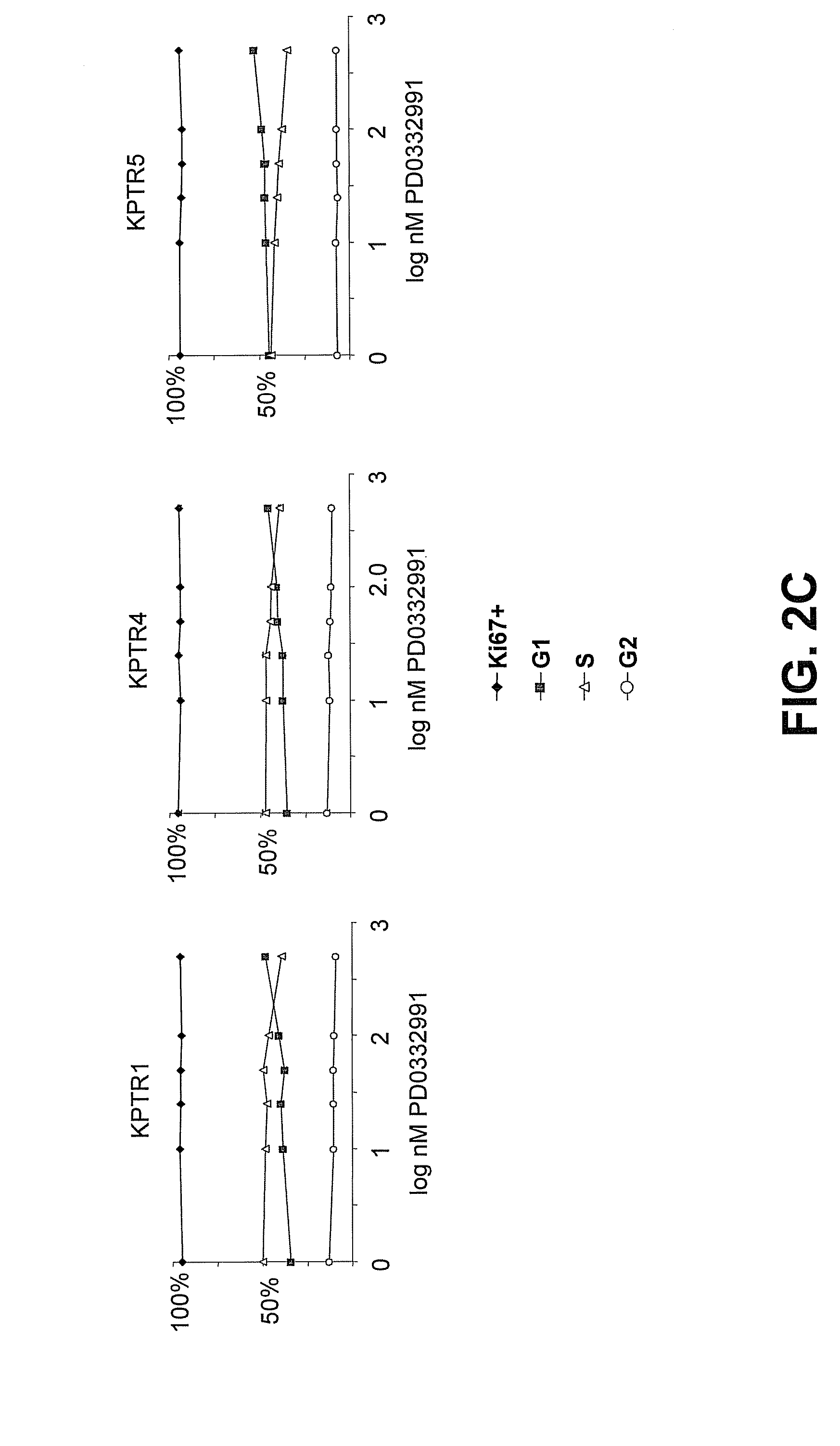
Hematopoietic protection against ionizing radiation using selective cyclin-dependent kinase 4/6 inhibitors
InactiveUS20110224221A1Reducing or preventing the effects of ionizing radiation on healthy cellsHigh activityBiocideAntinoxious agentsGeneticsCyclin-dependent kinase 4
Methods for reducing or preventing the effects of ionizing radiation in healthy cells arc provided. The methods relate to the use of selective cyclin-dependent kinase (CDK) 4 / 6 inhibitors to induce transient quiescence in CDK4 / 6 dependent cells, such as hematopoietic stem cells and / or hematopoietic progenitor cells. Radioprotection can be effected in mammals by treatment with selective CDK4 / 6 inhibitor compounds either before, at the same time as, or after exposure to the ionizing radiation.
Owner:NORTH CAROLINA AT CHAPEL HILL THE UNIV OF
Transient protection of normal cells during chemotherapy
This invention is in the area of improved compounds, compositions and methods of transiently protecting healthy cells, and in particular hematopoietic stem and progenitor cells (HSPC) as well as renal cells, from damage associated with DNA damaging chemotherapeutic agents. In one aspect, improved protection of healthy cells is disclosed using disclosed compounds that act as highly selective and short, transiently-acting cyclin-dependent kinase 4 / 6 (CDK 4 / 6) inhibitors when administered to subjects undergoing DNA damaging chemotherapeutic regimens for the treatment of proliferative disorders.
Owner:G1 THERAPEUTICS INC
Transient Protection of Normal Cells During Chemotherapy
This invention is in the area of improved compounds, compositions and methods of transiently protecting healthy cells, and in particular hematopoietic stem and progenitor cells (HSPC) as well as renal cells, from damage associated with DNA damaging chemotherapeutic agents. In one aspect, improved protection of healthy cells is disclosed using disclosed compounds that act as highly selective and short, transiently-acting cyclin-dependent kinase 4 / 6 (CDK 4 / 6) inhibitors when administered to subjects undergoing DNA damaging chemotherapeutic regimens for the treatment of proliferative disorders.
Owner:G1 THERAPEUTICS INC
Tricyclic Lactams for Use in the Protection of Normal Cells During Chemotherapy
InactiveUS20150297607A1Minimize impactAvoid selectivityOrganic active ingredientsOrganic chemistryProgenitorRegimen
This invention is in the area of tricyclic lactam compounds, compositions and methods of protecting healthy cells, and in particular hematopoietic stem and progenitor cells (HSPC) as well as renal cells, from damage associated with DNA damaging chemotherapeutic agents. In one aspect, protection of healthy cells is disclosed using compounds that act as cyclin-dependent kinase 4 / 6 (CDK 4 / 6) inhibitors when administered to subjects undergoing DNA damaging chemotherapeutic regimens for the treatment of proliferative disorders.
Owner:G1 THERAPEUTICS INC
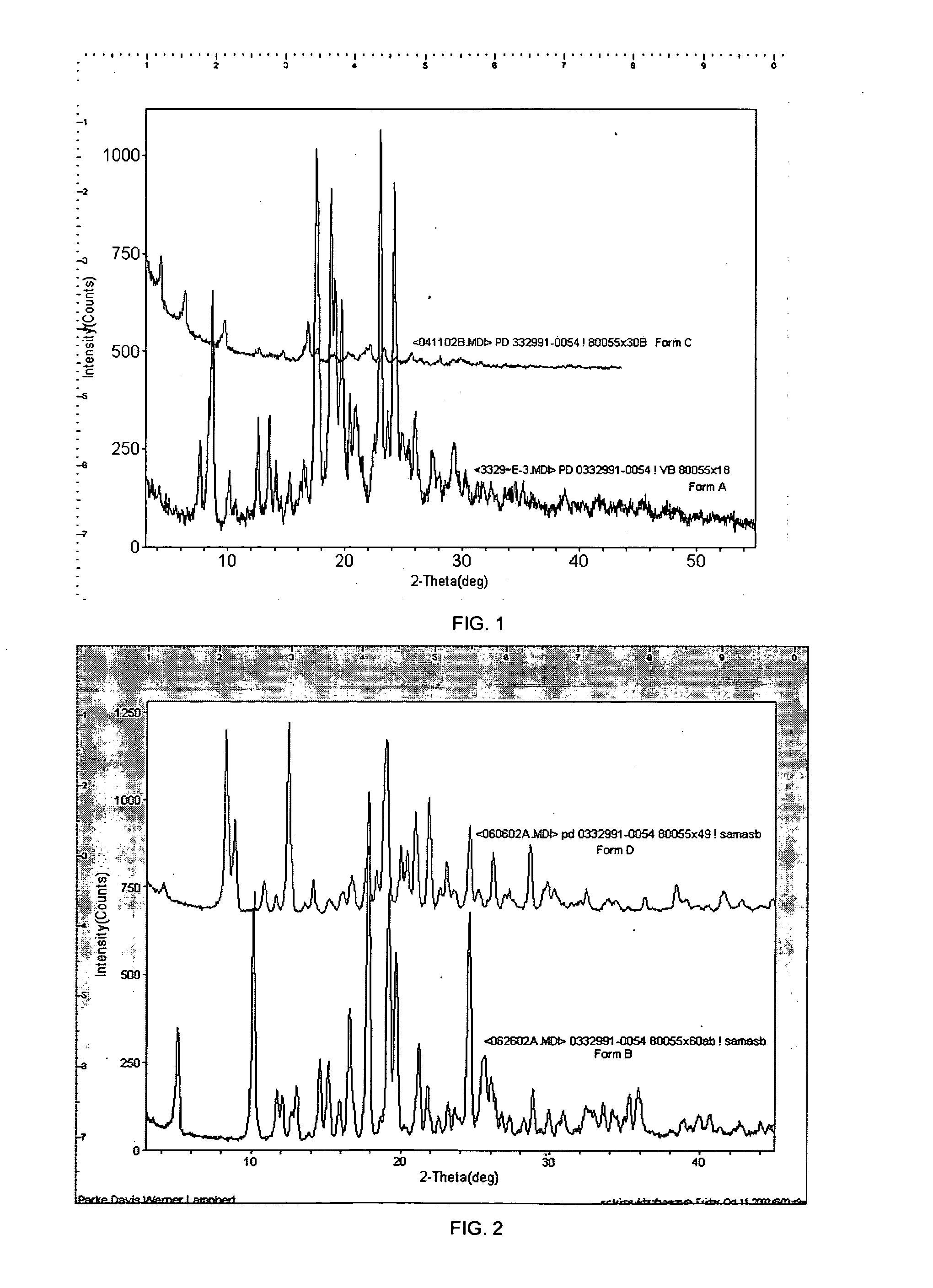
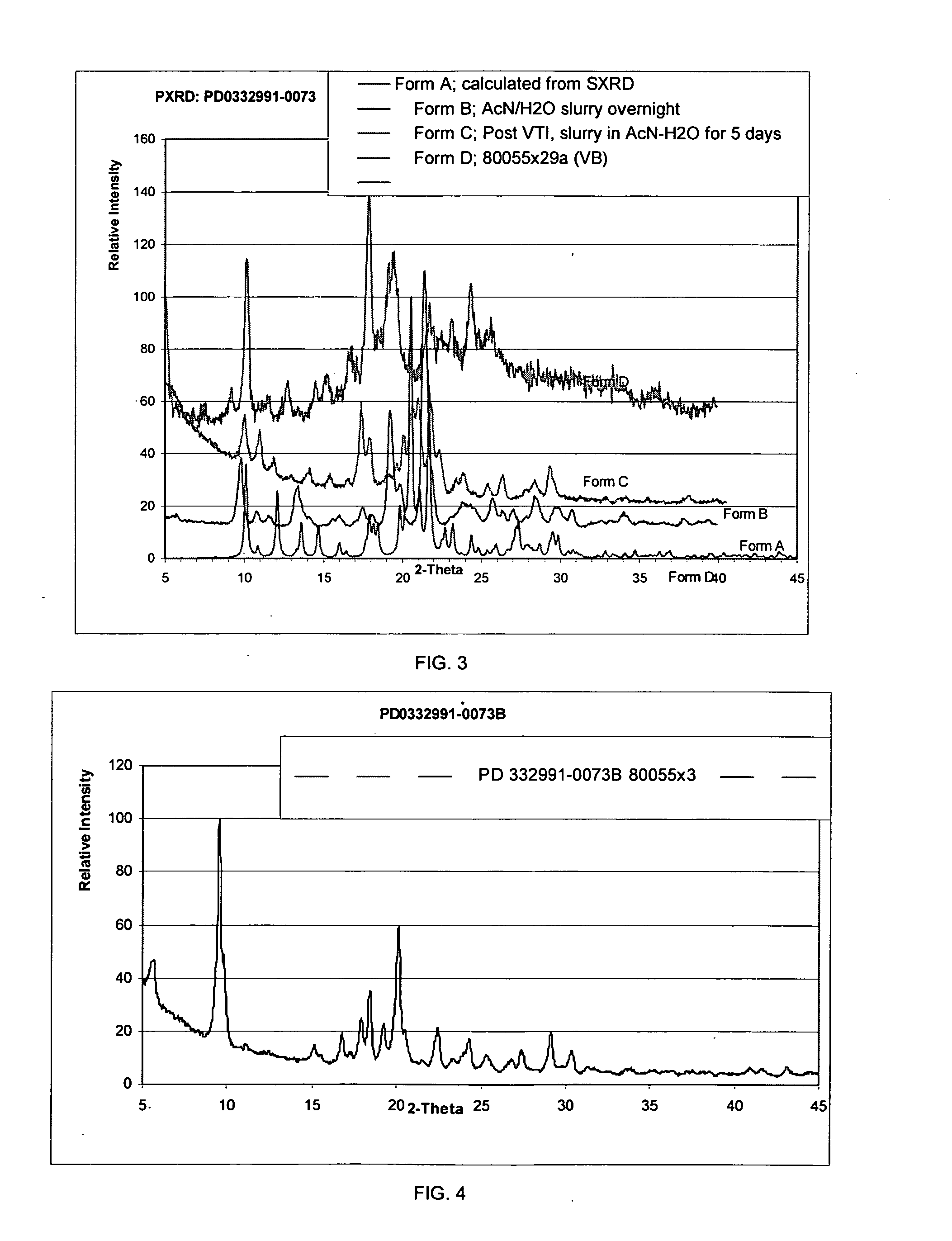
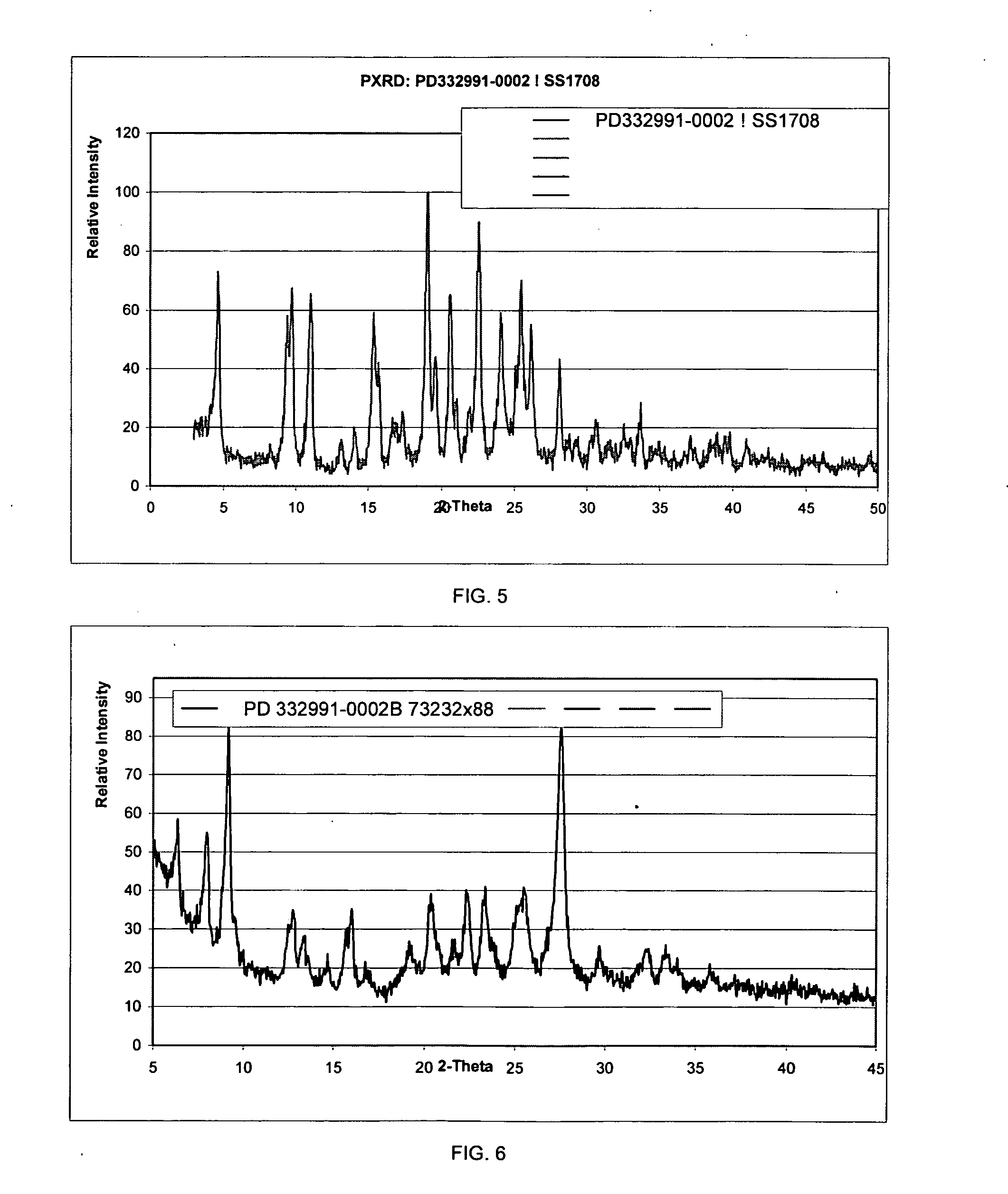
Isethionate salt of a selective CKD4 inhibitor
ActiveUS20050059670A1Increase productionLow hygroscopicityOrganic active ingredientsNervous disorderMedicinePercent Diameter Stenosis
Disclosed are polymorphs of the isethionate salt of 6-acetyl-8-cyclopentyl-5-methyl-2-(5-piperazin-1-yl-pyridin-2-ylamino)-8H-pyrido[2,3-d]pyrimidin-7-one, which is a selective cyclin-dependent kinase 4 (CDK4) inhibitor useful for treating inflammation and cell proliferative diseases such as cancer and restenosis.
Owner:WARNER LAMBERT CO LLC
Tricyclic Lactams for Use in HSPC-Sparing Treatments for RB-Positive Abnormal Cellular Proliferation
ActiveUS20150299212A1Rapid reentryFast rebuildOrganic active ingredientsOrganic chemistryProgenitorMedicine
This invention is in the area of tricyclic lactam compounds for and methods of treating selected Rb-positive cancers and other Rb-positive abnormal cellular proliferative disorders while minimizing the deleterious effects on healthy cells, for example healthy Hematopoietic Stem Cells and Progenitor Cells (HSPCs), associated with current treatment modalities. In one aspect, treatment of select Rb-positive cancers is disclosed using specific compounds disclosed herein. In certain embodiments, the compounds described herein act as selective cyclin-dependent kinase 4 / 6 (CDK 4 / 6) inhibitors when administered to subjects.
Owner:G1 THERAPEUTICS INC
Pyrazolopyrimidines
InactiveUS20050277655A1Useful in treatmentBiocideOrganic active ingredientsCyclin-dependent kinase 4Cyclin
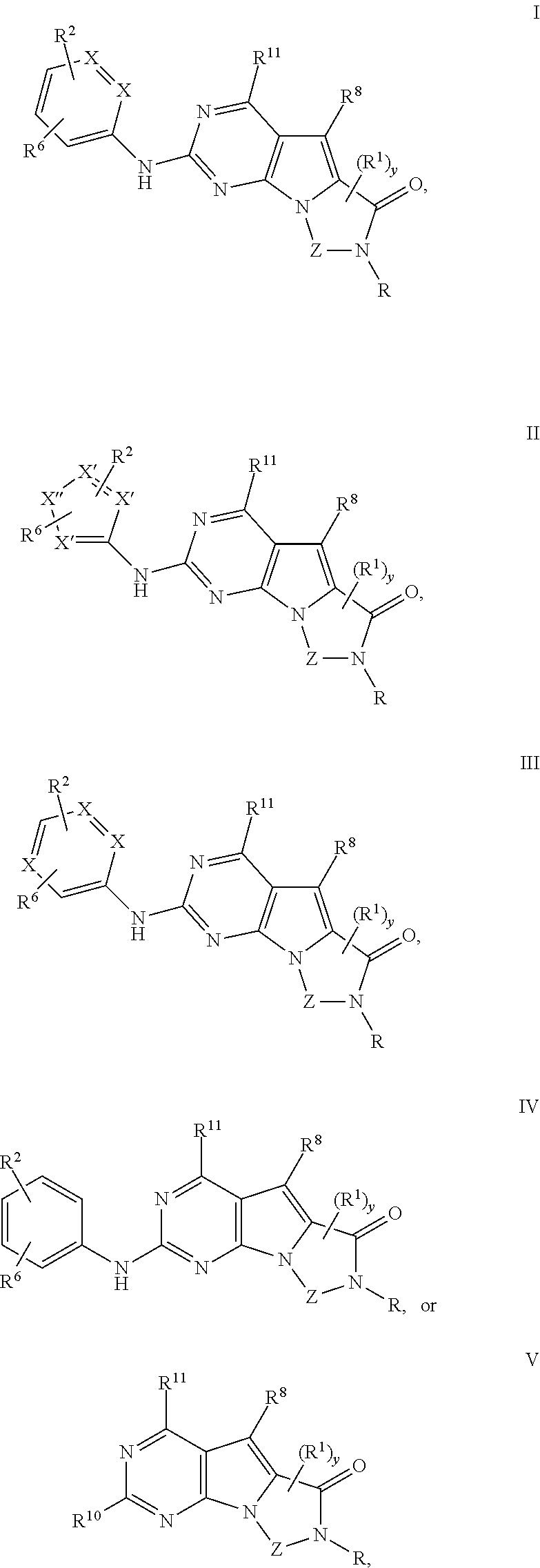
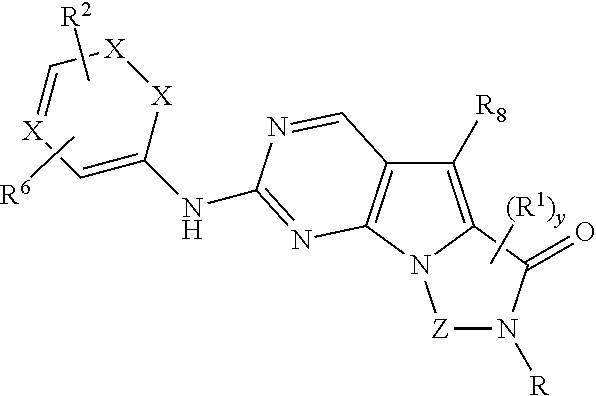
Novel pyrazolopyrimidines of formula (I): are discussed. These pyrazolopyrimidines are capable of inhibiting the activity of cyclin-dependent kinases, most particularly cyclin-dependent kinase 1 (Cdk1), cyclin-dependent kinase 2 (Cdk2), and cyclin-dependent kinase 4 (Cdk4) and are thus useful, inter alia, in the treatment or control of cancer, in particular solid tumors. This invention also provides pharmaceutical compositions containing such compounds and to methods of treating or controlling cancer, most particularly the treatment or control of breast, lung, colon and prostate tumors.
Owner:F HOFFMANN LA ROCHE INC
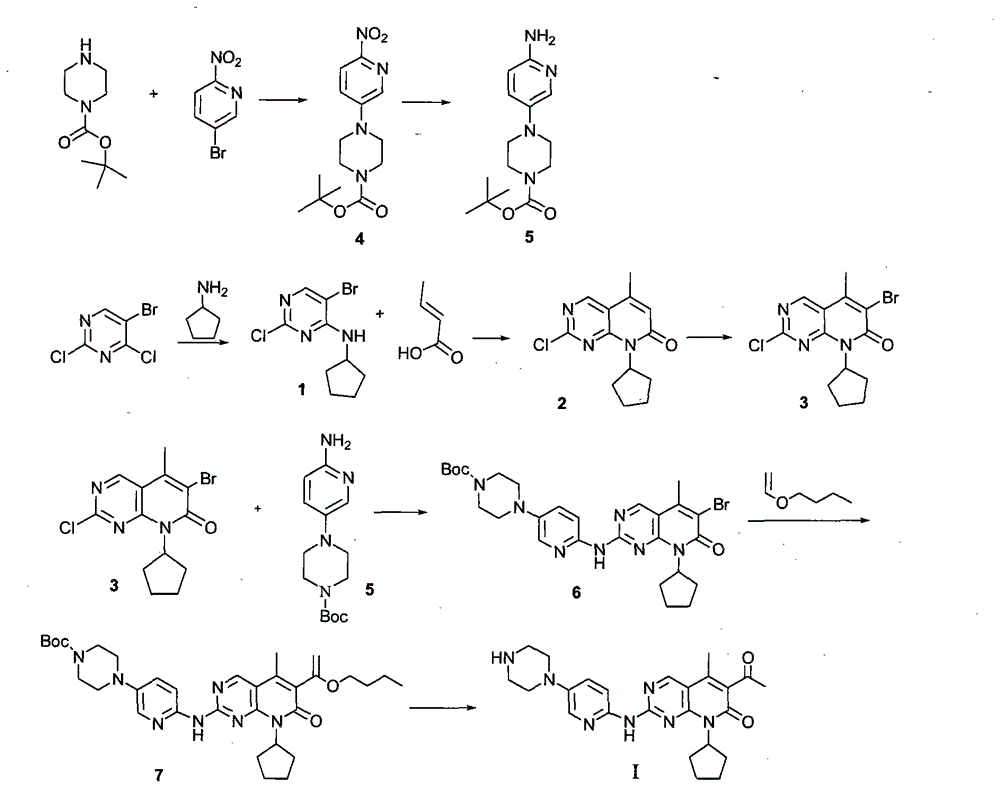
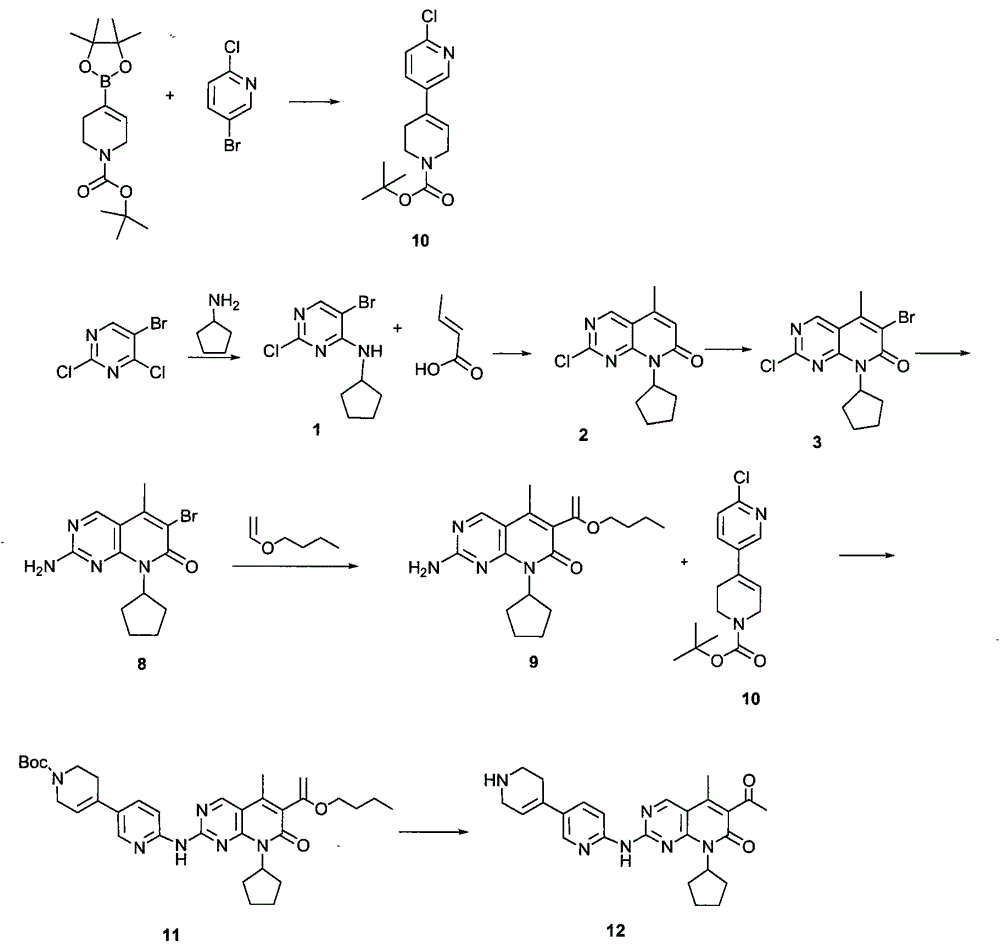
Novel synthesis method of CDK4 (cyclin-dependent kinase 4) inhibitor
ActiveCN104892604AMaintain blood levelsOrganic active ingredientsOrganic chemistrySynthesis methodsPyridine
The invention relates to a novel synthesis method of a CDK4 (cyclin-dependent kinase 4) inhibitor. A reaction of 2-chloro-8-cyclopentyl-5-methylpyrido[2,3-d]pyrimidin-7(8H)-one with acetylchloride is taken as an initial reaction, consequent reactions are performed, and the CDK4 inhibitor is prepared; the preparation method is simple and easy to implement, the yield is high, the quality is high, and industrial production is facilitated.
Owner:BEIJING KANG LISHENG PHARMA TECH DEV
Hematopoietic protection against chemotherapeutic compounds using selective cyclin-dependent kinase 4/6 inhibitors
Methods for reducing or preventing the effects of cytotoxic compounds in healthy cells are provided. The methods relate to the use of selective cyclin- dependent kinase (CDK) 4 / 6 inhibitors to induce transient quiescence in CDK4 / 6 dependent cells, such as hematopoietic stem cells and / or hematopoietic progenitor cells. Also described is a method of selecting compounds for reducing or preventing the effects of cytotoxic agents compounds in healthy cells.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Treatment Of RB-Negative Tumors Using Topoisomerase Inhibitors In Combination With Cyclin Dependent Kinase 4/6 Inhibitors
This invention is in the area of improved therapeutic combinations for and methods of treating selected retinoblastoma (Rb)-negative cancers and Rb-negative abnormal cellular proliferative disorders using particular topoisomerase inhibitors and specific cyclin-dependent kinase 4 / 6 (CDK4 / 6) inhibitors. In one aspect, the improved treatment of select Rb-negative cancers is disclosed using specific compounds disclosed herein in combination with a topoisomerase I inhibitor.
Owner:G1 THERAPEUTICS INC
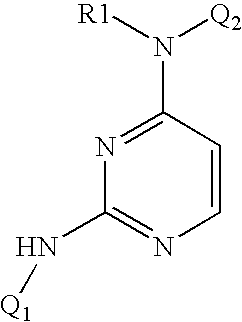
4-Aminopyrimidine-5-one derivatives
Novel 4-aminopyrimidine-5-one derivatives are disclosed. These compounds inhibit cyclin-dependent kinases, in particular cyclin-dependent kinase 4 (Cdk4). These compounds and their pharmaceutically acceptable salts and esters have antiproliferative activity and are useful in the treatment or control of cancer, in particular solid tumors. This invention is also directed to pharmaceutical compositions containing such compounds and to methods of treating or controlling cancer, most particularly the treatment or control of breast, lung, colon and prostate tumors. Also disclosed are intermediates useful in the preparation of these novel 4-aminopyrimidine-5-one derivatives.
Owner:F HOFFMANN LA ROCHE INC
Tricyclic lactams for use in HSPC-sparing treatments for RB-positive abnormal cellular proliferation
ActiveUS9717735B2Deleterious effectReduce anemiaOrganic active ingredientsOrganic chemistryProgenitorMedicine
This invention is in the area of tricyclic lactam compounds for and methods of treating selected Rb-positive cancers and other Rb-positive abnormal cellular proliferative disorders while minimizing the deleterious effects on healthy cells, for example healthy Hematopoietic Stem Cells and Progenitor Cells (HSPCs), associated with current treatment modalities. In one aspect, treatment of select Rb-positive cancers is disclosed using specific compounds disclosed herein. In certain embodiments, the compounds described herein act as selective cyclin-dependent kinase 4 / 6 (CDK 4 / 6) inhibitors when administered to subjects.
Owner:G1 THERAPEUTICS INC
Combination comprising a cyclin dependent kinase 4 or cyclin dependent kinase (cdk4/6) inhibitor for treating cancer
Owner:NOVARTIS AG
Treatment of rb-negative tumors using topoisomerase inhibitors in combination with cyclin dependent kinase 4/6 inhibitors
This invention is in the area of improved therapeutic combinations for and methods of treating selected retinoblastoma (Rb)-negative cancers and Rb-negative abnormal cellular proliferative disorders using particular topoisomerase inhibitors and specific cyclin-dependent kinase 4 / 6 (CDK4 / 6) inhibitors. In one aspect, the improved treatment of select Rb-negative cancers is disclosed using specific compounds disclosed herein in combination with a topoisomerase I inhibitor.
Owner:G1 THERAPEUTICS INC
Combination Comprising a Cyclin Dependent Kinase 4 or Cyclin Dependent Kinase (CDK4/6) Inhibitor for Treating Cancer
InactiveUS20160008367A1Organic active ingredientsAntineoplastic agentsCyclin-dependent kinase 4Cancer research
Owner:NOVARTIS AG
Application of pyrilamine compound as cycle protein dependency kinase 4/6 inhibitor
The invention relates to application of a pyrilamine compound as a cycle protein dependency kinase 4 / 6 inhibitor. The compound is of a structure shown as formula (I), preferably, wherein R1 and R2 are respectively hydrogen, hydroxyl or alkyl, and X is halogen. More preferably, R1 is hydrogen or hydroxyl, R2 is alkyl, X is fluorine, chlorine or bromine; and particularly preferably, R1 is hydrogen, R2 is methyl, ethyl, propyl, isopropyl, normal-butyl, isobutyl or tertiary butyl, and X is fluorine.
Owner:扬州市三药制药有限公司
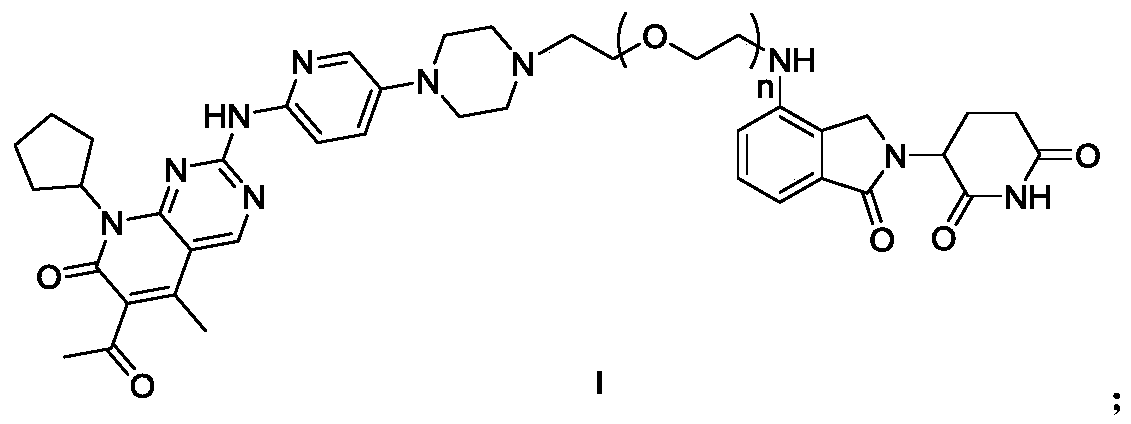
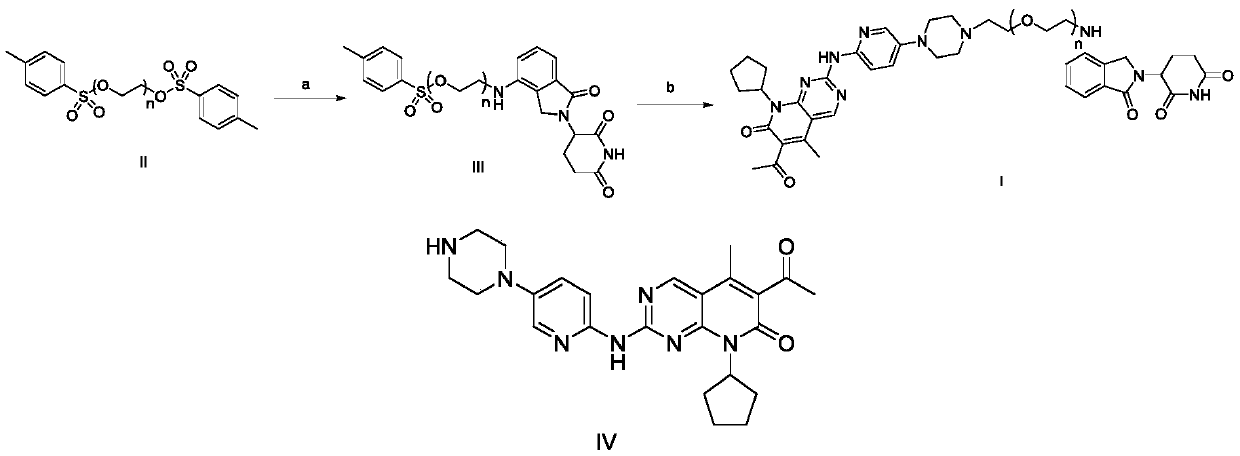
Compound to induce degradation of CDK4/6 (cyclin dependent kinase 4/6) based on CRBN (cereblon) ligand, preparation method of compound, pharmaceutical composition and application of compound
PendingCN110305126AGood antitumor activityEliminate side effectsOrganic active ingredientsOrganic chemistryCereblonSide effect
The invention discloses a compound to induce degradation of CDK4 / 6 (cyclin dependent kinase 4 / 6) based on CRBN (cereblon) ligand. A compound shown as formula I and a pharmaceutically acceptable salt and hydrate thereof are included herein. The invention also discloses a preparation method of the compound, a pharmaceutical composition of the compound, and application of the compound and the pharmaceutical composition in the preparation of drugs to prevent or / and treat cancers. Degradation of CDK4 / 6 can be induced just with a low quantity of the compound, and toxic and side effects upon the human body can be alleviated; excellent CDK4 / 6 degrading action and anticancer activity can be exhibited; the anticancer effect is better than that of a CDK4 / 6 inhibitor; the compound herein is suitable for preventing or / and treating various cancers and has a great application prospect in the field of medicine.
Owner:ZHEJIANG ACAD OF MEDICAL SCI
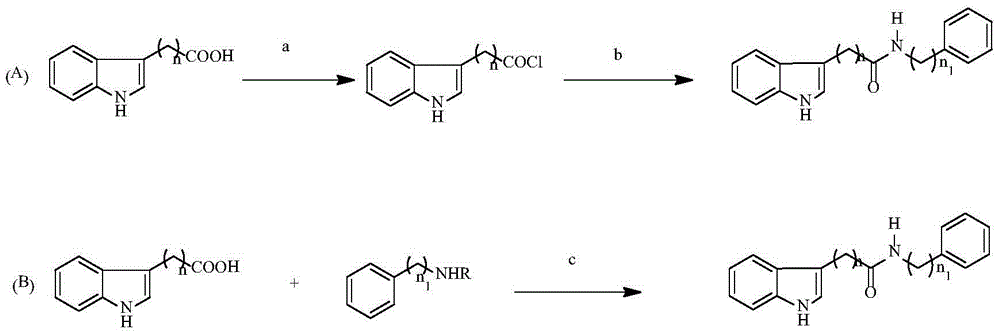
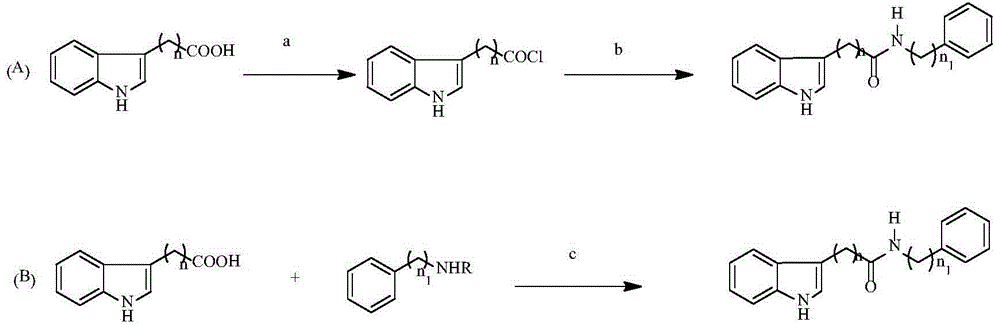
Indolylcarboxylic acid compound and application thereof in preparation of antitumor drugs
InactiveCN105777608AReduce planarityEmbedding capability decreasedOrganic active ingredientsOrganic chemistryChemical compoundCyclin-dependent kinase 4
The invention relates to the field of pharmaceutical chemistry and discloses an indolylcarboxylic acid compound represented by a formula (I) shown in the description and an application thereof in the preparation of antitumor drugs. The indolylcarboxylic acid compound disclosed by the invention plays a role in inhibiting cyclin-dependent kinase 4 and can be used for preparing the antitumor drugs and providing more pharmaceutical choices for tumor treatment.
Owner:LANZHOU UNIVERSITY
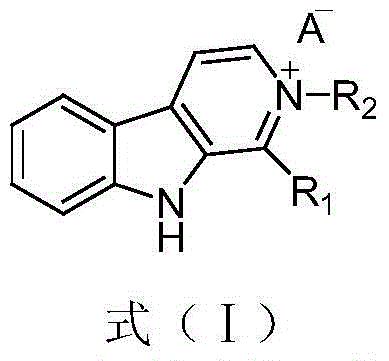
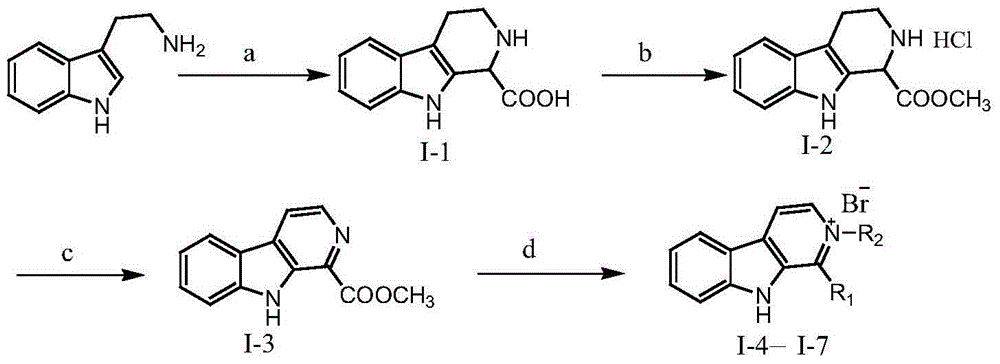
Beta-carboline alkaloid and application thereof in preparation of antitumor drugs
InactiveCN105753860AEnhanced inhibitory effectLow toxicityOrganic active ingredientsOrganic chemistryMedicineCyclin-dependent kinase 4
The invention relates to the field of pharmaceutical chemistry, and discloses a beta-carboline alkaloid represented by formula (I), and an application thereof in the preparation of antitumor drugs. The beta-carboline alkaloid has an inhibition effect on cyclin-dependent kinase 4, can be used to prepare the antitumor drugs, and provides more medicine choices for tumor treatment.
Owner:LANZHOU UNIVERSITY
Application of trichostatin A (TSA) to preparation of medicament for inhibiting activities of swine ovary granular cells
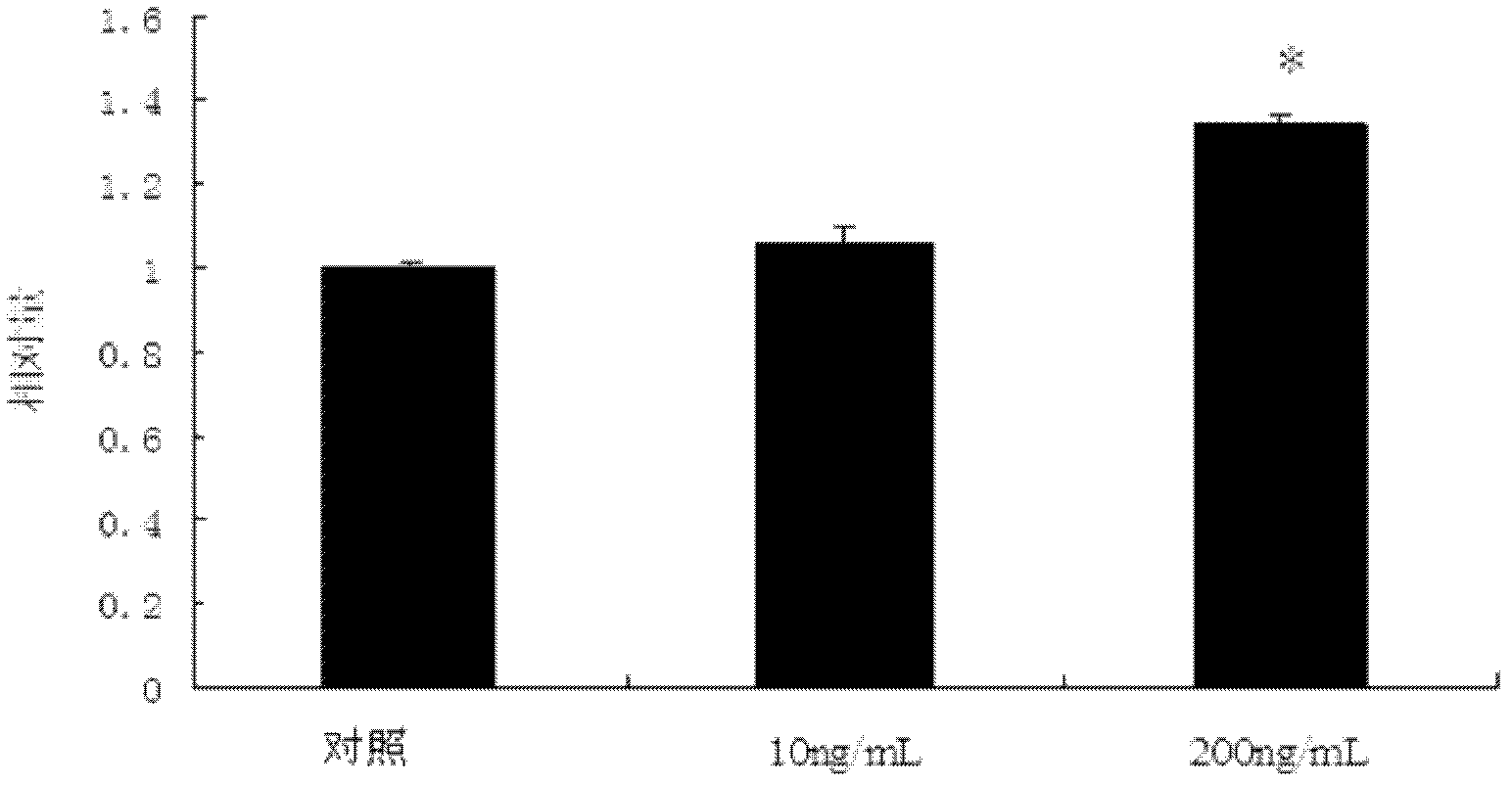
The invention belongs to the field of medicaments and veterinary medicines and discloses application of trichostatin A (TSA) to preparation of a medicament for inhibiting activity of swine ovary granular cells. In the invention, the influence of the TSA to the activities of the swine ovary granular cells is systemically studied, and a result shows that the TSA has no influence to the growth of the swine ovary granular cells at low concentration (below 10ng / mL), with the increase of the concentration, the growth of the swine ovary granular cells is inhibited (P<0.05), and the inhibition effect is dose-dependent. After the granular cells are treated by using 200ng / mL TSA, the cell cycle is retardant within a period G0 / G1, and the marked decrease of gene expression of Cyclin D2 and CDK4 (Cyclin-Dependent Kinase 4) is possible to be an important reason for the cycle retardance. Accordingly, the TSA can be applied to preparation of the medicament for inhibiting the activities of the swine ovary granular cells.
Owner:NANJING AGRICULTURAL UNIVERSITY
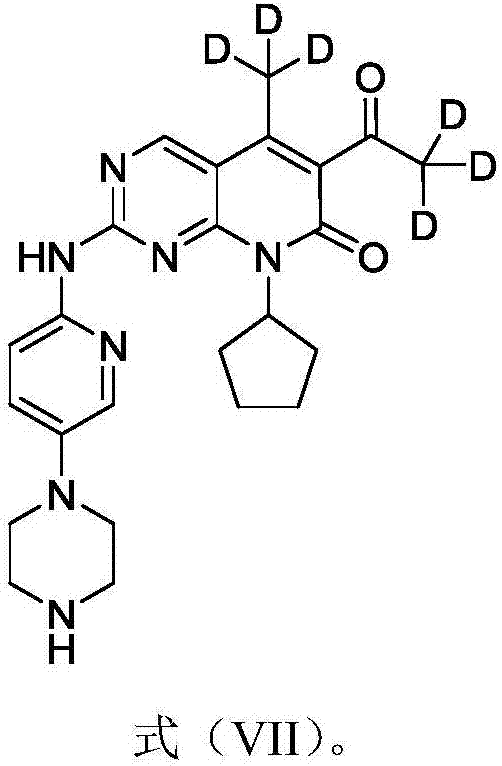
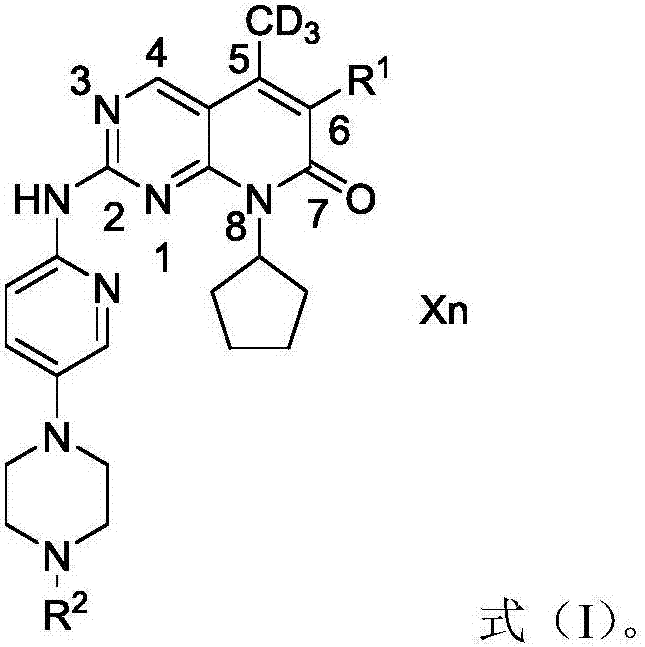
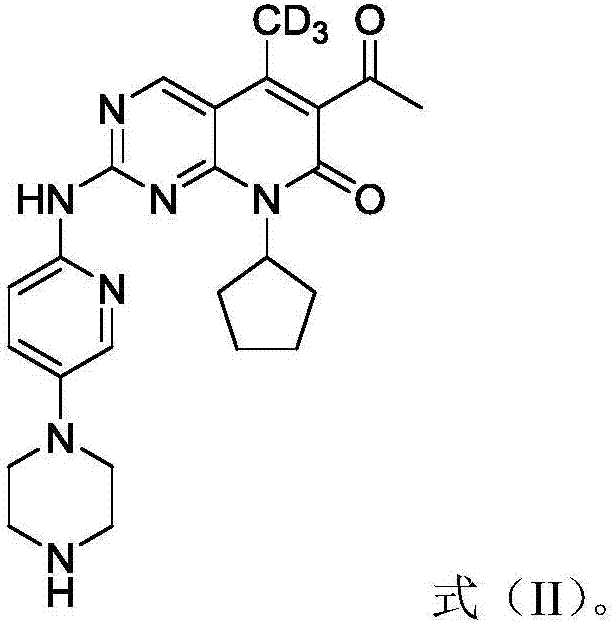
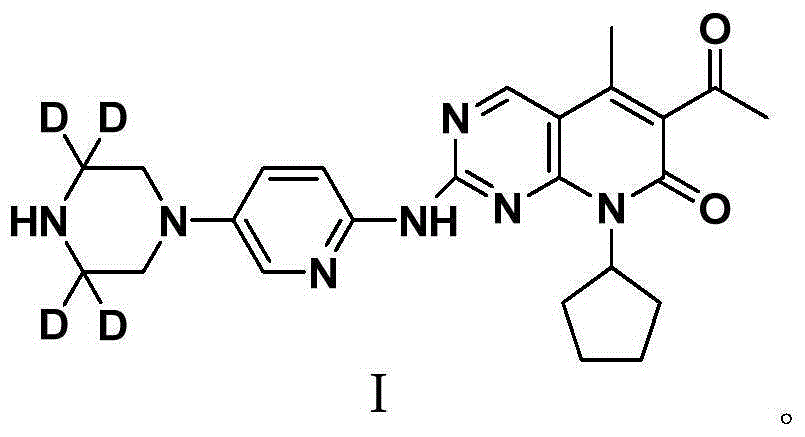
Deuterated palbociclib derivative and preparation method and application thereof
ActiveCN106967064AImprove pharmacokinetic propertiesGood curative effectIsotope introduction to heterocyclic compoundsAntineoplastic agentsTherapeutic effectCyclin-dependent kinase 4
The invention belongs to the technical field of pharmaceutical compounds and particularly relates to a deuterated palbociclib derivative and preparation method and application thereof; the deuterated palbociclib derivative has a structure shown as in formula (I); by selectively deuterating active metabolic sites of palbociclib, the metabolic nature of a pharmaceutical is improved, and the therapeutic effect, safety and durability of the pharmaceutical are improved accordingly; through the synthesis of the deuterated palbociclib derivative, a novel compound is provided for synthesizing a novel antitumor pharmaceutical; this compound is applicable to the inhibitor pharmaceutical aspect to inhibit cyclin dependent kinase 4 and / or 6, and the aspect of pharmaceuticals for treating breast cancer, ovarian cancer, liver cancer or acute lymphoblastic leukemia.
Owner:TYK MEDICINES INC
Deuterated Palbociclib crystal
InactiveCN106432223AHigh purityHigh crystallinityOrganic chemistry methodsAntineoplastic agentsCrystallinityCyclin-dependent kinase 4
The invention provides a deuterated Palbociclib crystal shown in the formula I and a preparation method and pharmaceutical application thereof and particularly relates to a deuterated Palbociclib crystal shown in the formula I, a preparation method of the crystal, a crystal composition containing the crystal, a pharmaceutical composition containing the crystal and the crystal composition thereof and pharmaceutical application of the crystal and the crystal composition thereof. The crystal has the advantages of being high in purity and crystallinity, good in stability and easy to prepare, and can be used for preparing medicine for treating diseases caused by cyclin dependent kinase 4 and 6. The formula I is shown in the specification.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Pharmaceutical combination comprising the PI3K inhibitor alpelisib and the CDK4/6 inhibitor ribociclib, and the use thereof in the treatment/prevention of cancer
InactiveUS10328066B2Inhibit progressAvoid symptomsAntineoplastic agentsHeterocyclic compound active ingredientsCancer preventionRibociclib
The present disclosure pertains to a pharmaceutical combination comprising (a) an alpha-isoform specific PI3K inhibitor, (b) a cyclin dependent kinase 4 / 6 (CDK4 / 6) inhibitor, and (c) an antimetabolite antineoplastic agent; combined preparations and pharmaceutical compositions thereof; the uses of such a combination in the treatment or prevention of cancer; and methods of treating or preventing cancer in a subject comprising administering a therapeutically effective amount of such combination.
Owner:NOVARTIS AG
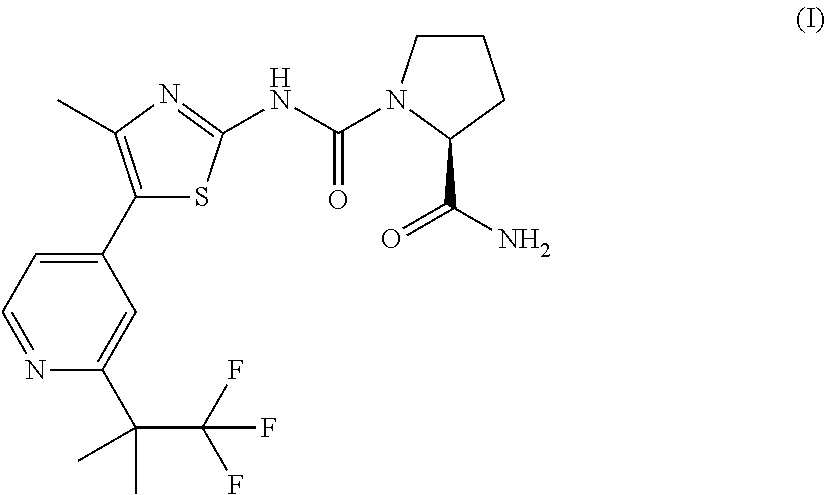
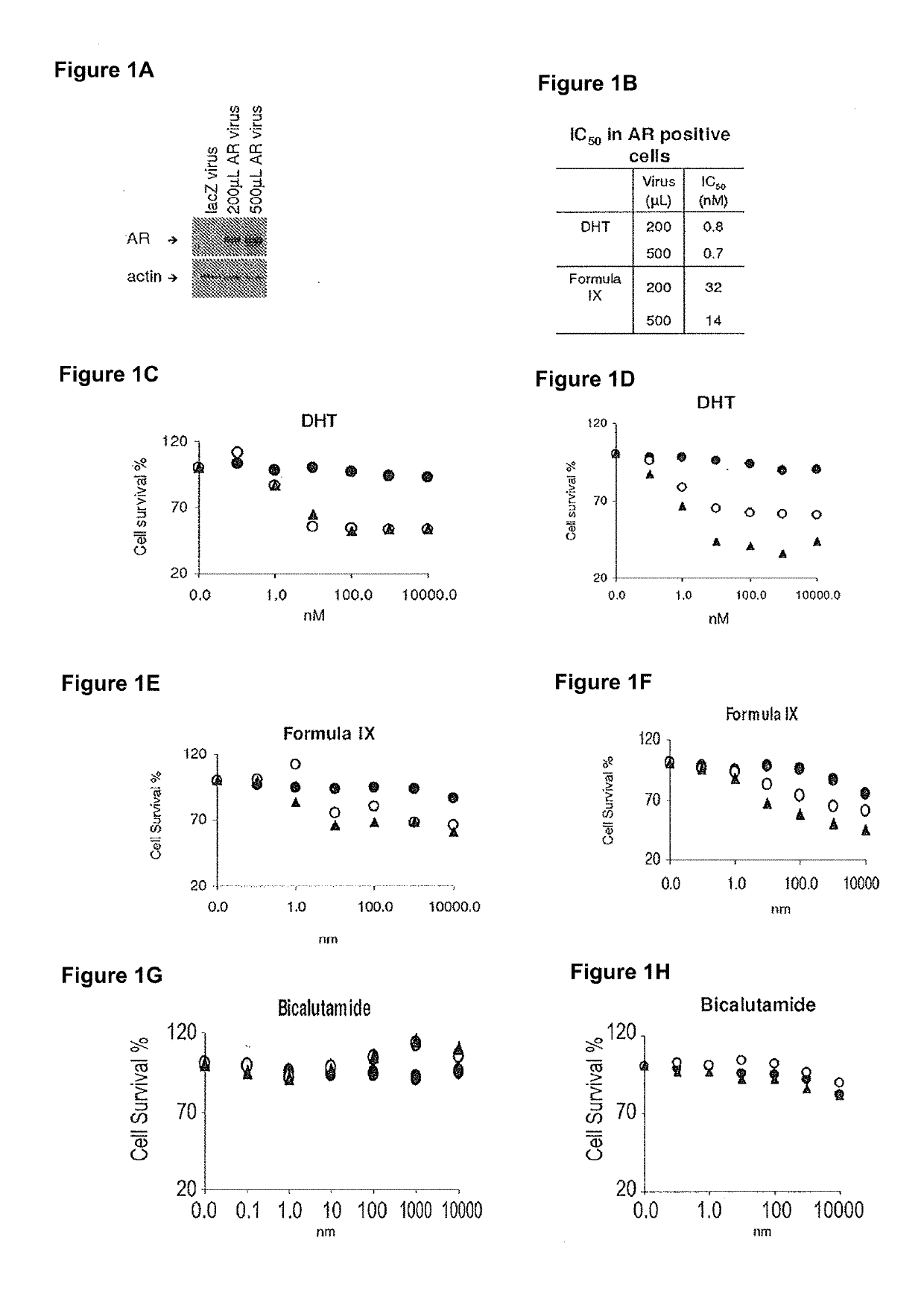
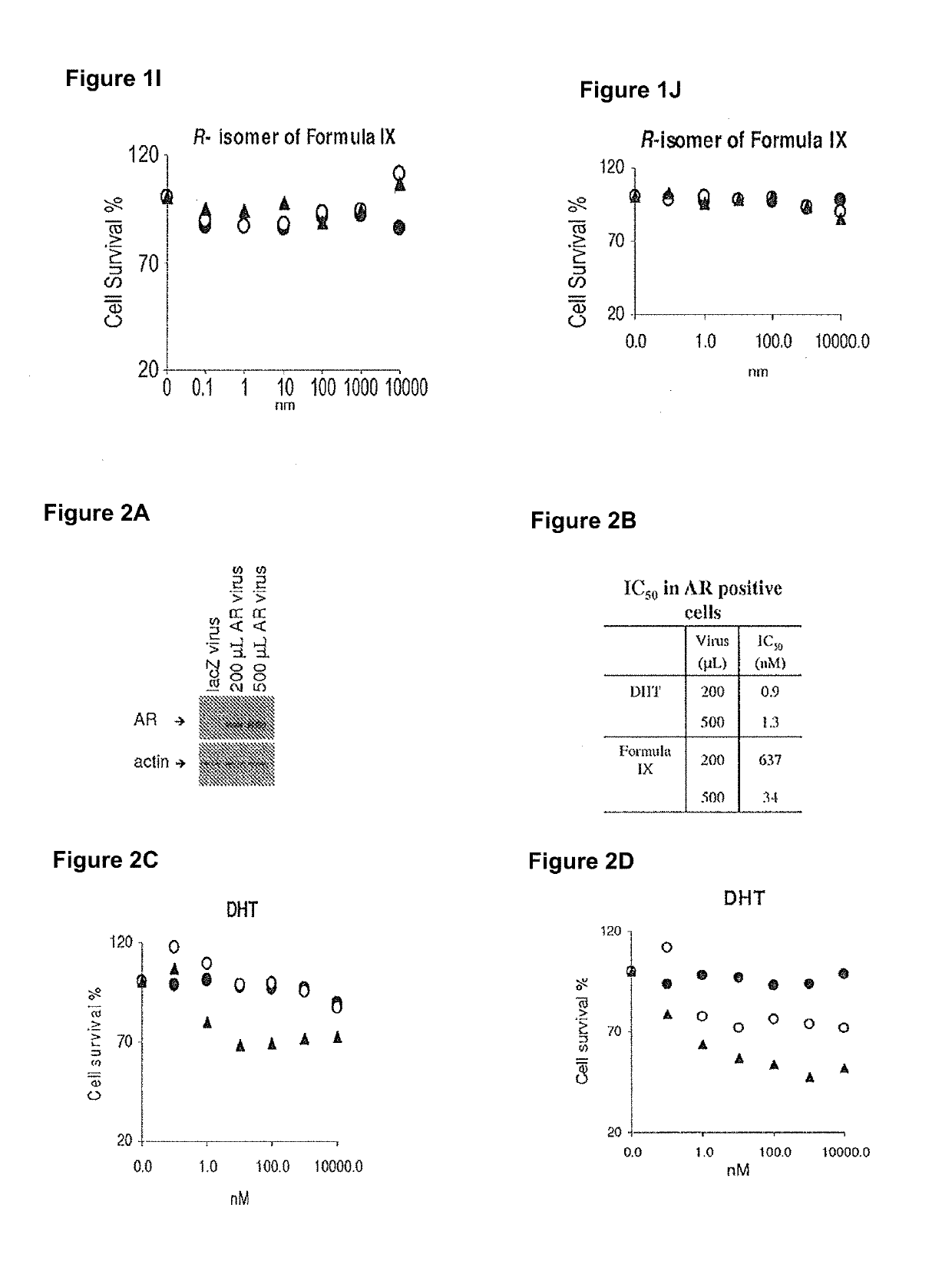
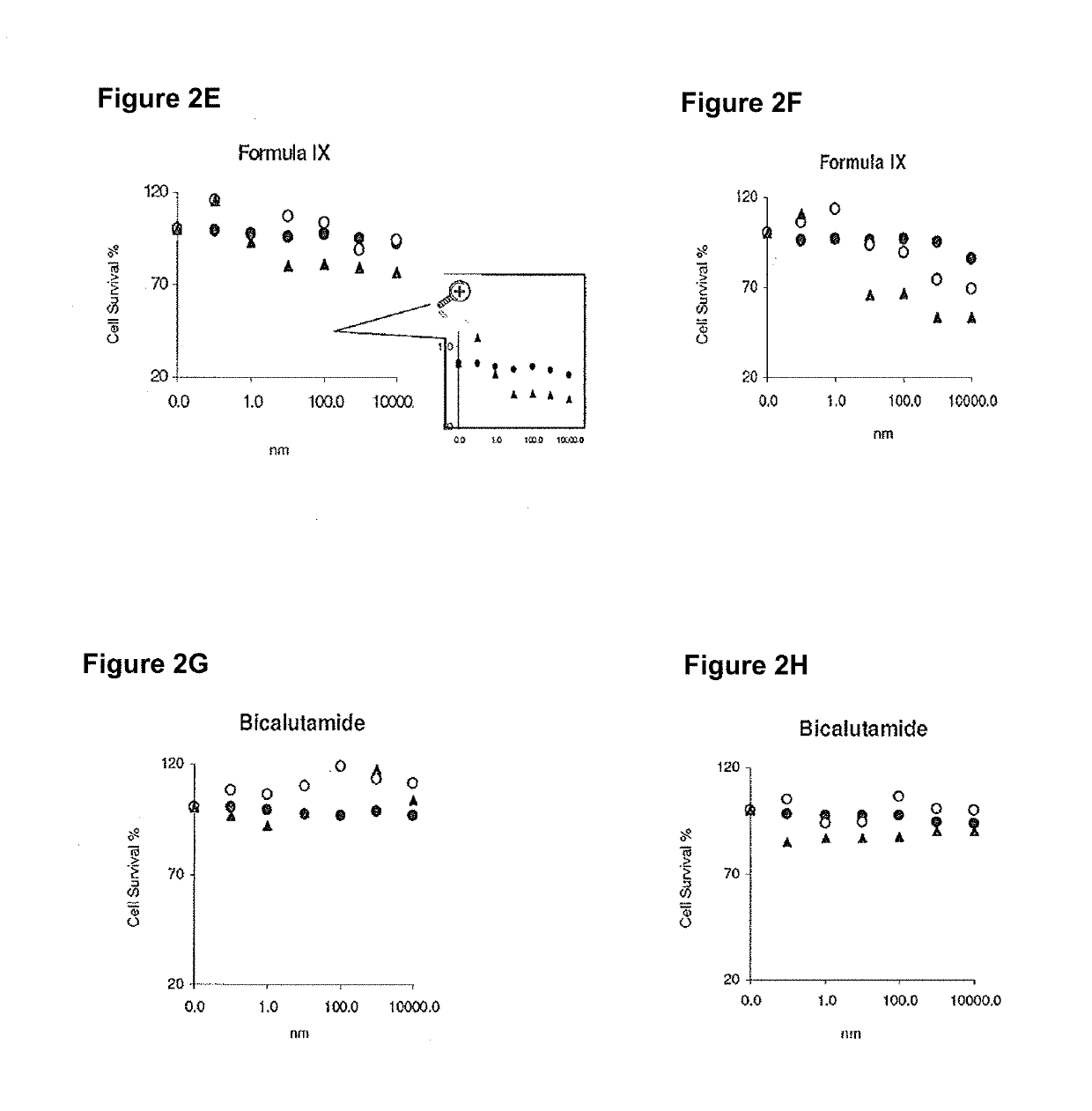
Method of treating er mutant expressing breast cancers with selective androgen receptor modulators (SARMS)
This invention relates to the treatment of breast cancer in a subject, for example a female subject. Including methods of: treating metastatic breast cancer; refractory breast cancer; AR-positive breast cancer; AR-positive refractory breast cancer; AR-positive metastatic breast cancer; AR-positive and ER-positive breast cancer; triple negative breast cancer; advanced breast cancer; breast cancer that has failed selective estrogen receptor modulator (SERM) (tamoxifen, toremifene, raloxifene), gonadotropin-releasing hormone (GnRH) agonist (goserelin), aromatase inhibitor (AI) (letrozole, anastrozole, exemestane), cyclin-dependent kinase 4 / 6 (CDK 4 / 6) inhibitor (palbociclib (Ibrance), ribociclib (Kisqali), abemaciclib (Vorzenio)), mTOR inhibitor (everolimus), trastuzumab (Herceptin, ado-trastuzumab emtansine), pertuzumab (Perjeta), lapatinib, neratinib (Nerlynx), olaparib (Lynparza) (an inhibitor of the enzyme poly ADP ribose polymerase (PARP)), bevacizumab (Avastin), and / or fulvestrant treatments; metastasis in a subject suffering from breast cancer; HER2-positive; and / or treating a subject suffering from ER mutant expressing breast cancer, comprising administering to the subject a therapeutically effective amount of a selective androgen receptor modulator (SARM) compound.
Owner:UNIV OF TENNESSEE RES FOUND
Crystallization of deuteration Palbociclib isethionate
InactiveCN106432224AOrganic active ingredientsIsotope introduction to heterocyclic compoundsCrystallinityCyclin-dependent kinase 4
The invention provides a crystallization, preparation method and medical application of a deuteration Palbociclib isethionate as shown in the formula I and particularly relates to the crystallization of the deuteration Palbociclib isethionate shown in equation I, a preparation method of the crystal, the crystal compositions containing the crystal, the medicine compositions containing the crystal and the crystal compositions, and the medical application of the crystal and the crystal compositions. The crystal has the advantages of having high purity and high crystallinity, having good stability and being easy to prepare. The crystal can be adopted for the preparation of drugs for the treatment of the diseases caused by cyclin dependent kinase 4 and 6. Please see the formula in the description.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
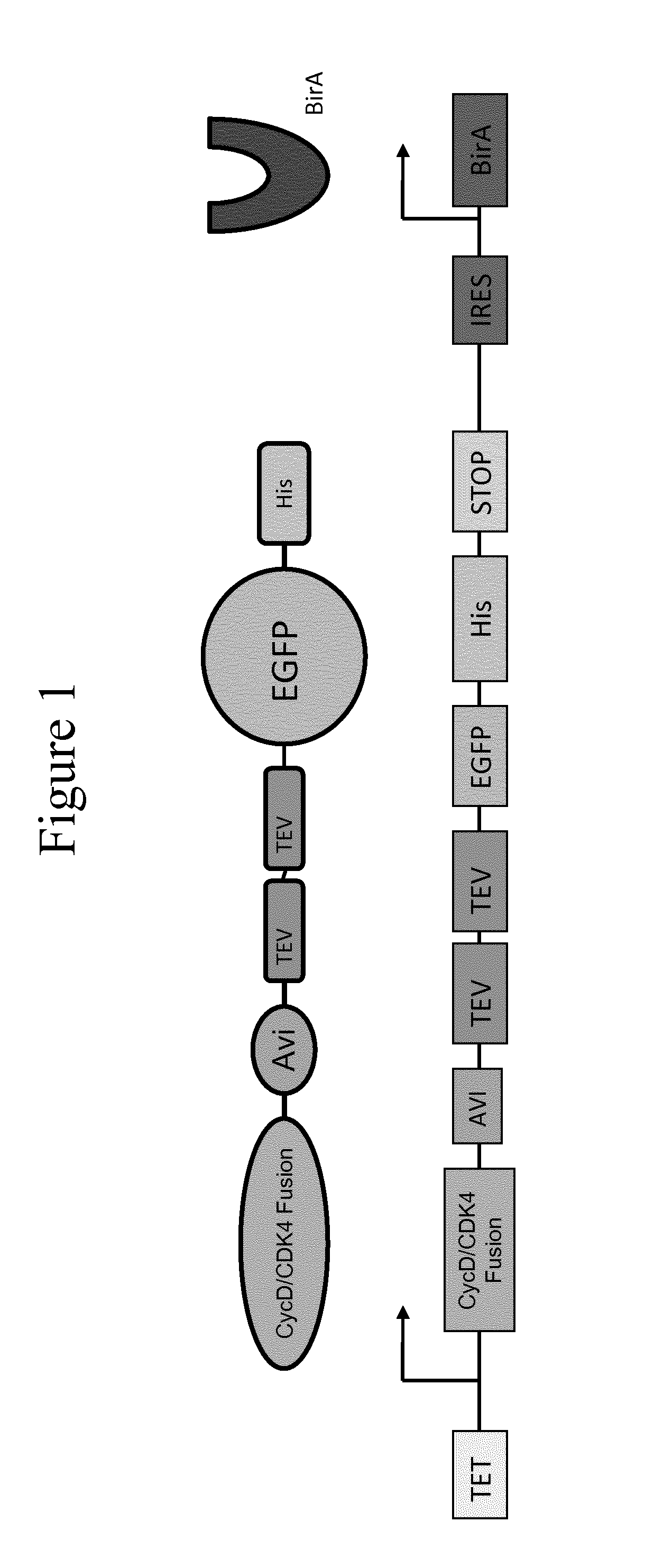
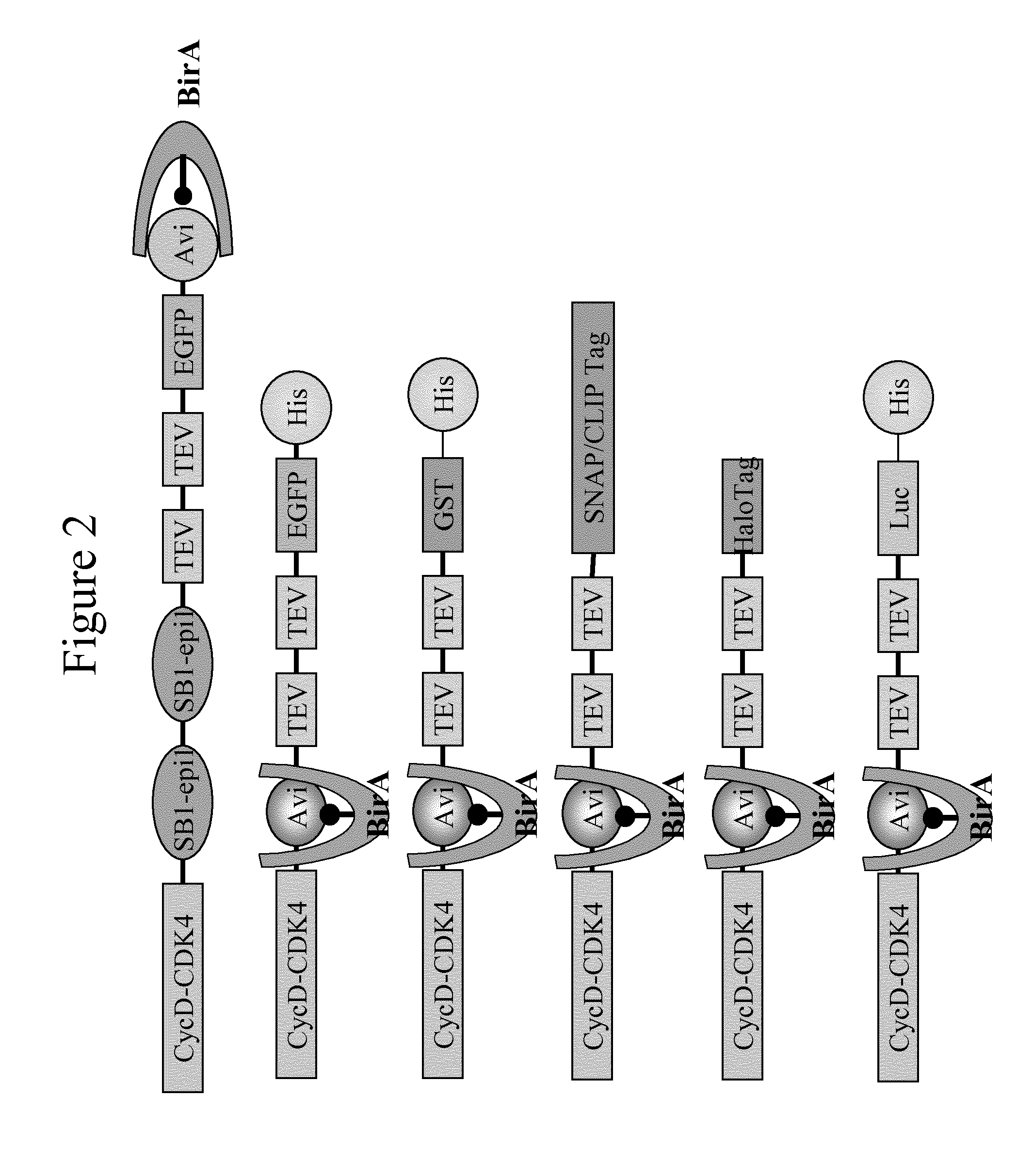
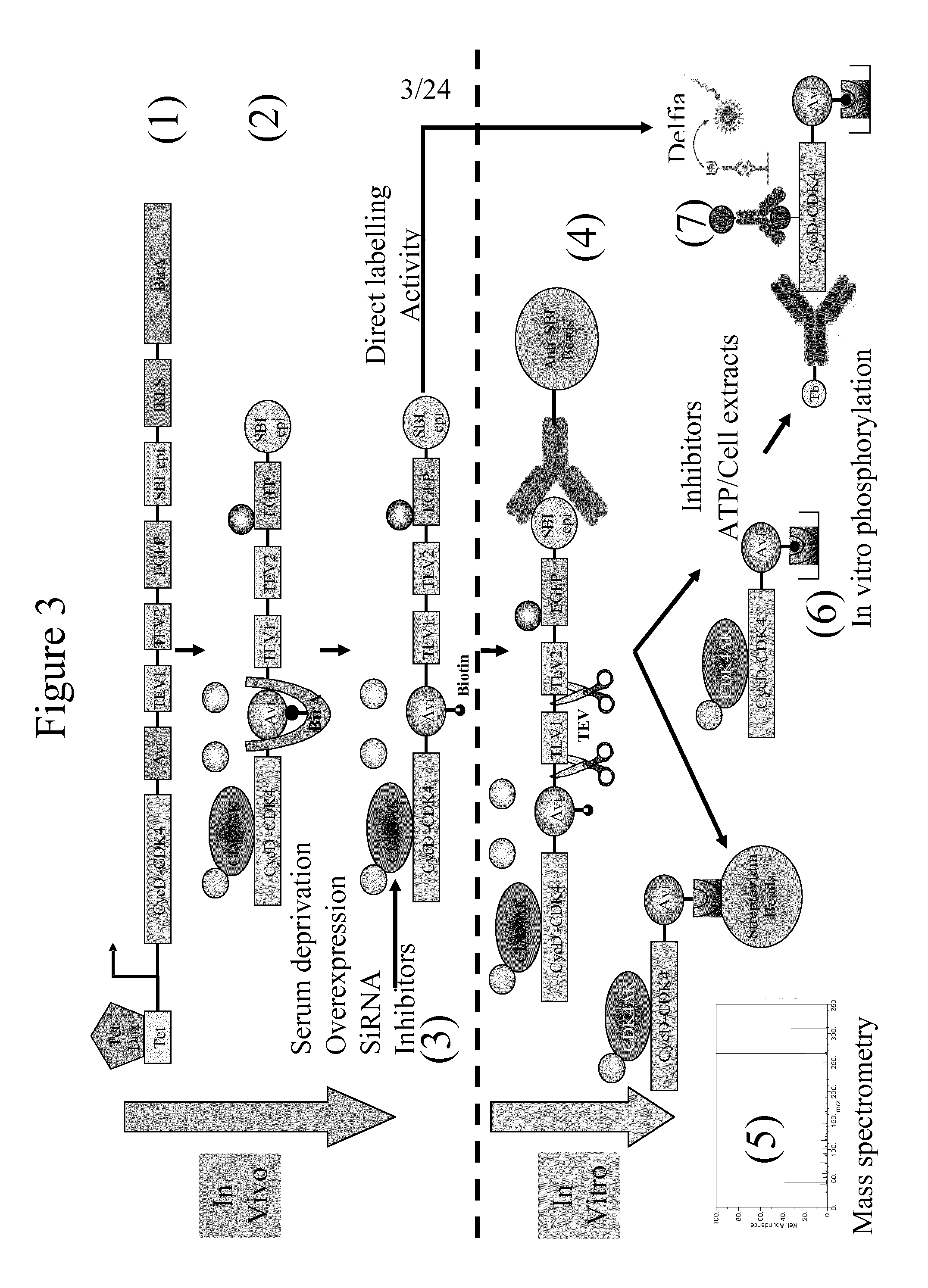
Immobilised cyclin-dependent kinase 4 fusion proteins and uses thereof
InactiveUS20150299764A1Reserved functionImprove throughputMicrobiological testing/measurementBiological material analysisPhosphorylationBiological activation
The present invention concerns an in vitro assay for determining the activation status of endogenous CDK4 in eukaryotic cells, said assay comprising the steps of: providing eukaryotic cells maintained in a quiescent state, said eukaryotic cells comprising a cyclin D / CDK4 fusion protein, wherein the CDK4 part of the fusion protein is present in a hypophosphorylated form; inducing proliferation of said eukaryotic cells; isolating the cyclin D / CDK4 fusion protein from said eukaryotic cells; and measuring the activation status of said isolated cyclin D / CDK4 fusion protein, thereby determining the activation stats of endogenous CDK4 in said eukaryotic cells.
Owner:UNIV LIBRE DE BRUXELIES
Non-invasive method of evaluating breast cancers for selective androgen receptor modulator (SARM) therapy
This invention relates to the treatment of breast cancer in a subject, for example a female subject. Including methods of: treating metastatic breast cancer; refractory breast cancer; AR-positive breast cancer; AR-positive refractory breast cancer; AR-positive metastatic breast cancer; AR-positive and ER-positive breast cancer; triple negative breast cancer; advanced breast cancer; breast cancer that has failed selective estrogen receptor modulator (SERM) (tamoxifen, toremifene, raloxifene), gonadotropin-releasing hormone (GnRH) agonist (goserelin), aromatase inhibitor (AI) (letrozole, anastrozole, exemestane), cyclin-dependent kinase 4 / 6 (CDK 4 / 6) inhibitor (palbociclib (Ibrance), ribociclib (Kisqali), abemaciclib (Vorzenio)), mTOR inhibitor (everolimus), trastuzumab (Herceptin, ado-trastuzumab emtansine), pertuzumab (Perjeta), lapatinib, neratinib (Nerlynx), olaparib (Lynparza) (an inhibitor of the enzyme poly ADP ribose polymerase (PARP)), bevacizumab (Avastin), and / or fulvestrant treatments; metastasis in a subject suffering from breast cancer; HER2-positive; treating a subject suffering from ER mutant expressing breast cancer and / or treating breast cancer in a subject, by first determining the 18F-16β-fluoro-5α-dihydrotestosterone (18F-DHT) tumor uptake and identifying said subject as having AR-positive breast cancer based on 18F-DHT tumor uptake, comprising administering to the subject a therapeutically effective amount of a selective androgen receptor modulator (SARM) compound.
Owner:UNIV OF TENNESSEE RES FOUND
Antitumor human single-chain antibody
ActiveCN102140139AImmunogenicAvoid side effectsAntibody ingredientsAntineoplastic agentsTumor targetSingle-Chain Antibodies
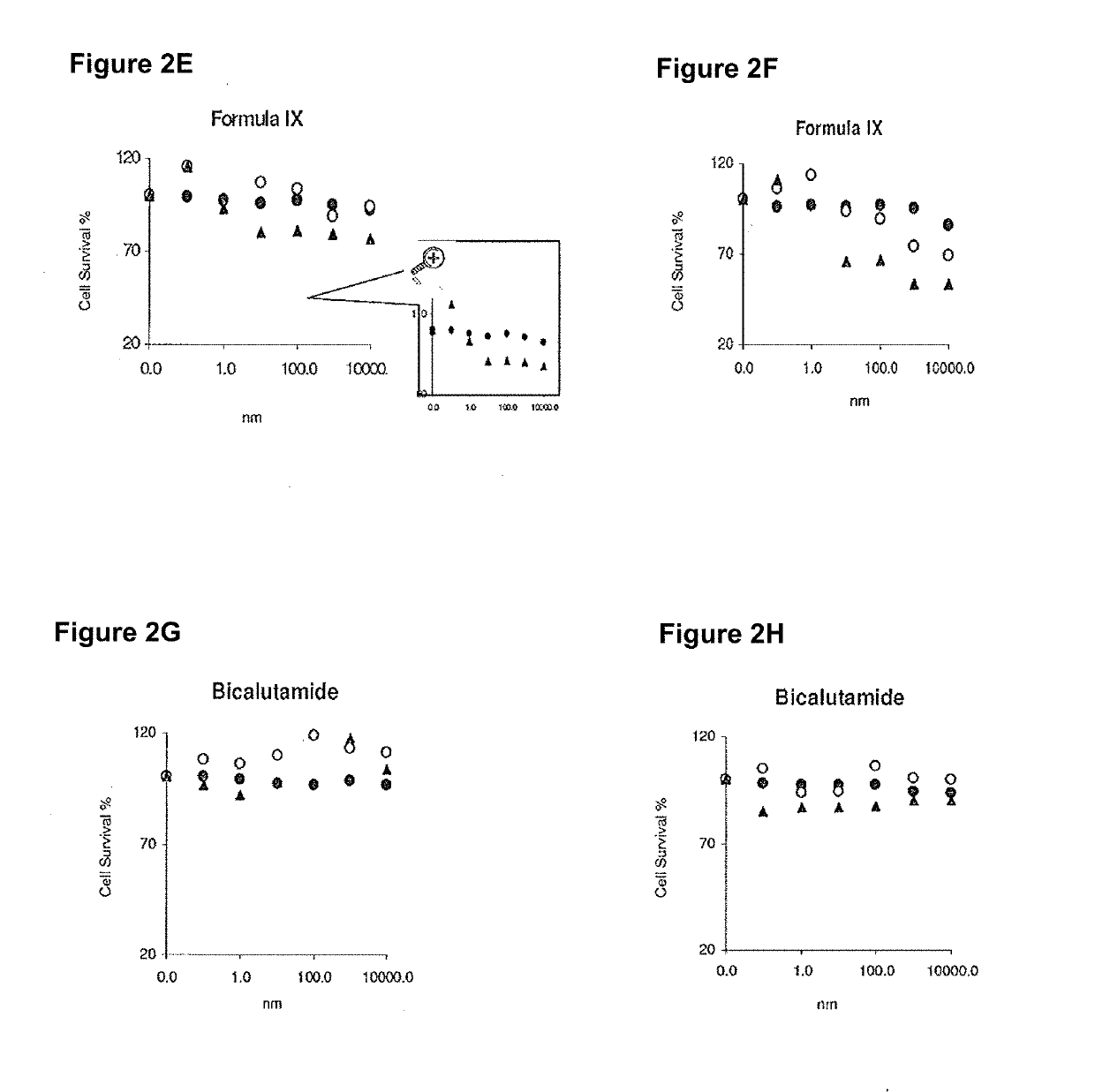
The invention relates to an antitumor human single-chain antibody, which belongs to a human antibody, in particular to a specific anti-human D1-cyclin-dependent kinase 4 (CDK4) human single-chain antibody which has a nucleotide sequence represented by a Sequence No.1. The antitumor human single-chain antibody also comprises genetic coding sequence of nuclear localization signal (NLS) peptide and an E-tag peptide, and the nucleotide sequence of the genetic coding sequence is represented by a Sequence No.2. The invention also relates to the use of the antitumor human single-chain antibody in the preparation of antitumor medicines, wherein the tumors treated are human breast cancer and human cervical carcinoma. The invention antitumor human single-chain antibody is a low-toxicity, high-efficiency and specific molecule for inhibiting the activity of CDK4 and has the advantages of small molecular weight, low immunogenicity and the like. A tumor specific eukaryotic expression vector is usedto perform the tumor targeted treatment of the antibody and realize the controllability of the therapeutic gene, and the tumor specific eukaryotic expression vector has tumor specificity and therefore ensures treatment safety and effectiveness.
Owner:JILIN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com