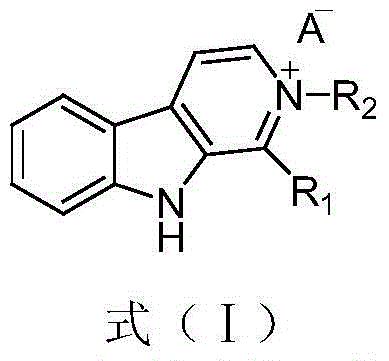
Beta-carboline alkaloid and application thereof in preparation of antitumor drugs
An alkaloid and carboline technology, applied in the field of cyclin-dependent kinase 4 inhibitors, can solve the problems of high toxicity and side effects, limited clinical application, high cytotoxicity, etc., and achieve the effect of low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1 Preparation of carboline alkaloids represented by formula (I)
[0037] 1. Instrument
[0038] (1) Melting point is measured with WRS-2 microcomputer melting point measuring instrument (Shanghai Shengyan Ultrasonic Instrument Co., Ltd.);
[0039] (2) IR × spectrum was measured with a NicoletImpact410 infrared spectrometer, and pressed into KBr tablets;
[0040](3) 1HNMR is measured with a JEOLFX90Q Fourier transform nuclear magnetic resonance instrument;
[0041] (4) MS was determined by Nicolet2000 Fourier transform mass spectrometer and MAT-212 mass spectrometer.
[0042] 2. Preparation method
[0043]
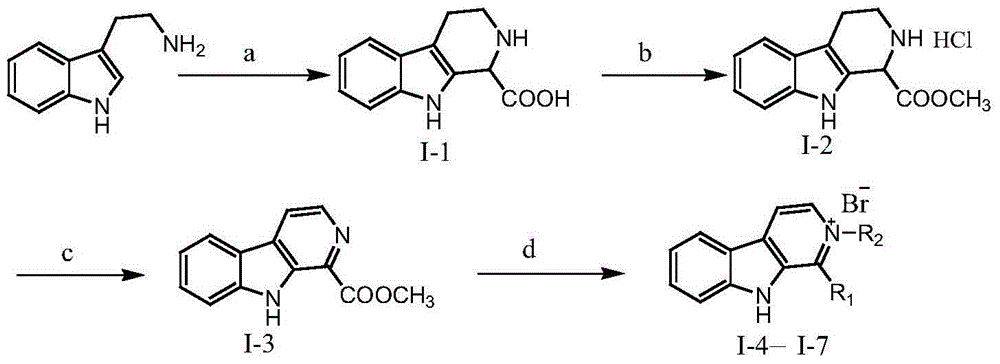
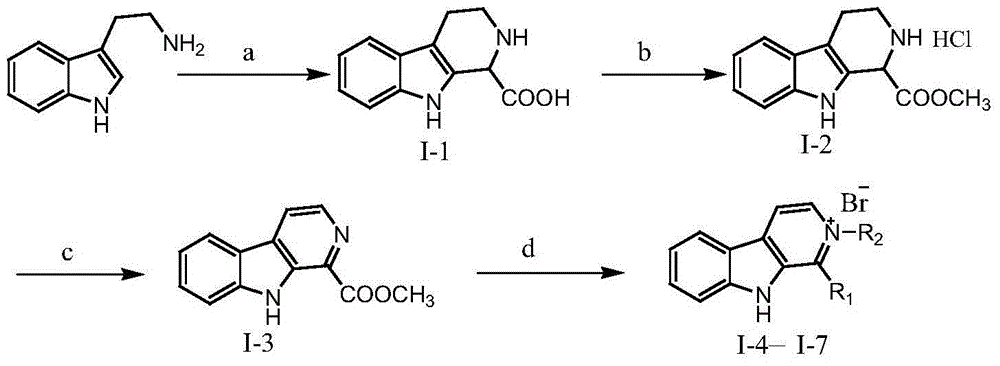
[0044] The preparation process of the carboline alkaloid of formula (I) is as follows:
[0045]
[0046] (1) 1-Formic acid-1,2,3,4-tetrahydro-β-carboline (Ⅰ-1)
[0047] Weigh 5.00g of tryptamine and place it in a 250mL three-necked bottle, add 60.00mL of water, add an appropriate amount of concentrated hydrochloric acid under stirring until the ...
Embodiment 2
[0076] Example 2 Toxicity assessment of carboline alkaloids represented by formula (I)
[0077]
[0078] Choose the 2-phenyl-1-formic acid methyl ester group-9H-β-carboline bromide (I-4) (R 1 = Methoxycarbonyl, R 2 = phenyl) and fascaplysin (formula (II)) were simulated by chem3D software for dihedral angle data, and then their planarity was compared.
[0079] Table 1 Comparison of the dihedral angle data of Fascaplysin and 2-phenyl-1-formyl-9H-β-carboline bromide salt
[0080]
[0081]
[0082] According to the data simulated by computer drug design software, the most planar 2-phenyl-1-formic acid methyl ester group-9H-β-carboline bromide (I-4) (R 1 = methyl formate, R 2 =Phenyl) structure is less planar than fascaplysin, but the planar structure of fascaplysin allows it to be embedded in DNA and has higher cytotoxicity.
[0083] Therefore, the designed compound of the present invention is more effective than 2-phenyl-1-formic acid methyl group-9H-β-carboline bro...
Embodiment 3
[0085] Example 3 Verification experiment of CDK4 inhibitory activity of carboline alkaloids represented by formula (I)
[0086] 1. Materials
[0087] Instrument TECAN Safire2 measuring instrument, black wall and black bottom 384-well plate (CORNING, USA), plate shaker (Jiangsu Guangming Experimental Instrument Factory), reagent CDK4 / clyclinD, pRb protein substrate, DMSO (Sigma)
[0088] 2. Experimental method
[0089] (1) Take 133ul of 5× buffer and add it to 367ul of water to obtain 500ul of 1.33× kinase buffer;
[0090] (2) Add 0.2ul of CDK4 / clyclinD and 0.8ul of substrate to 199ul of 1.33×kinase buffer to obtain 200ul of kinase / substrate mixture;
[0091] (3) Take 6ul10mMATP and add it to 144ul1.33 kinase buffer to obtain 150ul4×ATP solution;
[0092] (4) Add 0.2ul of phosphorylated peptide to 49.8ul of 1.33×kinase buffer to obtain 50ul of phosphorylated peptide solution;
[0093] (5) Take the synthetic compound Ⅰ-1——Ⅰ-7 and configure it into 2ul10 -2 The aqueous solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com