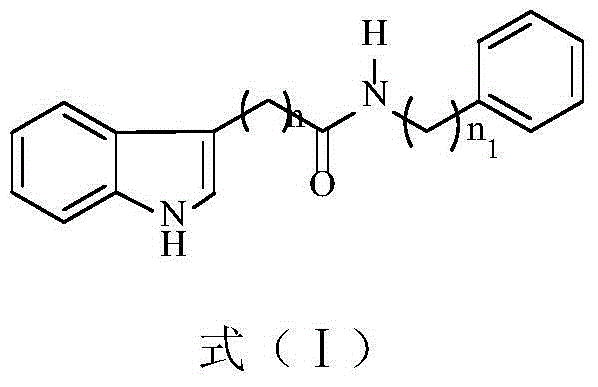
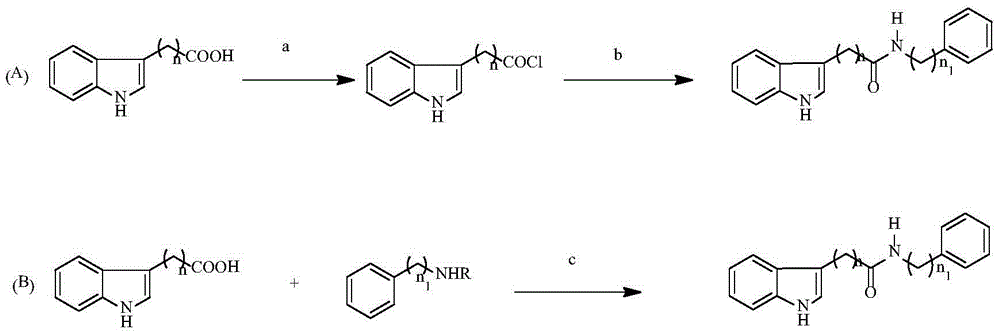
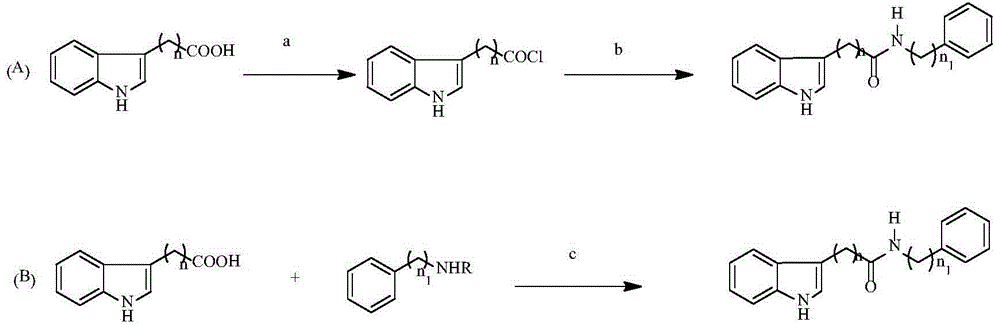
Indolylcarboxylic acid compound and application thereof in preparation of antitumor drugs
A technology of indole carboxylic acid and compound, which is applied in the field of cyclin-dependent kinase 4 inhibitor, can solve the problems of large toxic and side effects, limited clinical application, high cytotoxicity and the like, and achieves the effect of low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1 Preparation of indole carboxylic acid compounds as represented by formula (I)
[0026] 1. Instrument
[0027] (1) Melting point is measured with WRS-2 microcomputer melting point measuring instrument (Shanghai Shengyan Ultrasonic Instrument Co., Ltd.);
[0028] (2) The IR spectrum was measured with a NicoletImpact410 infrared spectrometer, and the KBr tablet was pressed;
[0029] (3) 1HNMR is measured with a JEOLFX90Q Fourier transform nuclear magnetic resonance instrument;
[0030] (4) MS was determined by Nicolet2000 Fourier transform mass spectrometer and MAT-212 mass spectrometer.
[0031] 2. Preparation method
[0032]
[0033] (1) 2-(3-indolyl)-N-phenylacetamide (Ⅰ-1)
[0034] Synthetic Route 1: Dissolve 5.00mmol of indole-3-acetic acid in 15.00mL of dry tetrahydrofuran, add SOCl dropwise in an ice-water bath 2 6.00mmol was added dropwise, 50 Flow for one hour, spin dry the solvent, and use it directly in the next step without purification. ...
Embodiment 2
[0052] Example 2 Toxicity assessment of indole carboxylic acid compounds represented by formula (I)
[0053]
[0054] In the present invention, the natural product Fascaplysin (planar pentacyclic structure parent nucleus) with specific and selective CDK4 small molecule inhibitory activity is used as the lead compound, and the ring-opening idea is used to retain its interaction site with CDK4, and the computer-aided drug design software is used to design A series of derivatives of non-planar specific fascaplysin were developed. Select planar maximum 2-(3-indolyl)-N-phenylacetamide (I-1) (n=1n 1 =0) and fascaplysin (formula (II)) were simulated by chem3D software for dihedral angle data, and then their planarity was compared.
[0055] Table 1 The dihedral angle data of fascaplysin and indole carboxylic acid compound I-1
[0056]
[0057] According to the data simulated by computer drug design software, the compound of the present invention has the largest 2-(3-indolyl)-N...
Embodiment 3
[0060] Example 3 Verification experiment of CDK4 inhibitory activity of indole carboxylic acid compounds represented by formula (I)
[0061] 1. Materials
[0062] Instrument TECAN Safire2 measuring instrument, black wall and black bottom 384-well plate (CORNING, USA), plate shaker (Jiangsu Guangming Experimental Instrument Factory), reagent CDK4 / clyclinD, pRb protein substrate, DMSO (Sigma)
[0063] 2. Experimental method:
[0064] (1) Take 133ul of 5× buffer and add it to 367ul of water to obtain 500ul of 1.33× kinase buffer;
[0065](2) Add 0.2ul of CDK4 / clyclinD and 0.8ul of substrate to 199ul of 1.33×kinase buffer to obtain 200ul of kinase / substrate mixture;
[0066] (3) Add 6ul10mMATP to 144ul1.33×kinase buffer to obtain 150ul4×ATP solution;
[0067] (4) Add 0.2ul of phosphorylated peptide to 49.8ul of 1.33×kinase buffer to obtain 50ul of phosphorylated peptide solution;
[0068] (5) Take 2ul10 -2 M mother solution was added to 498ul water to obtain 500ul4× test comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com