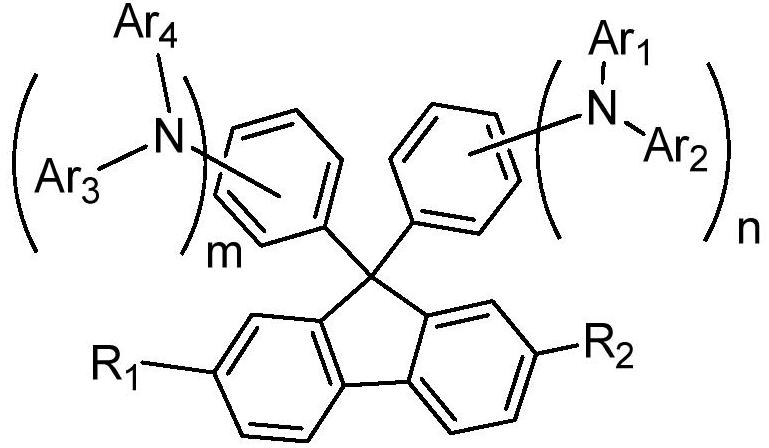
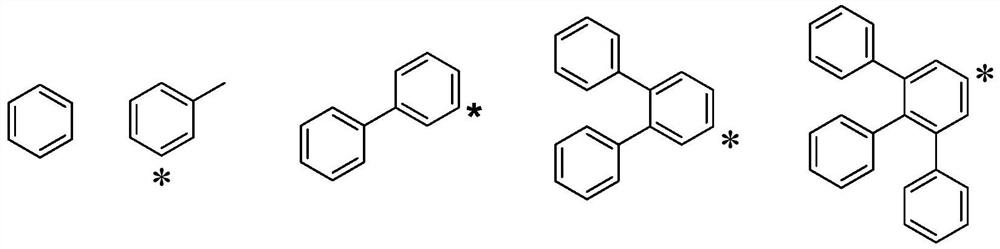
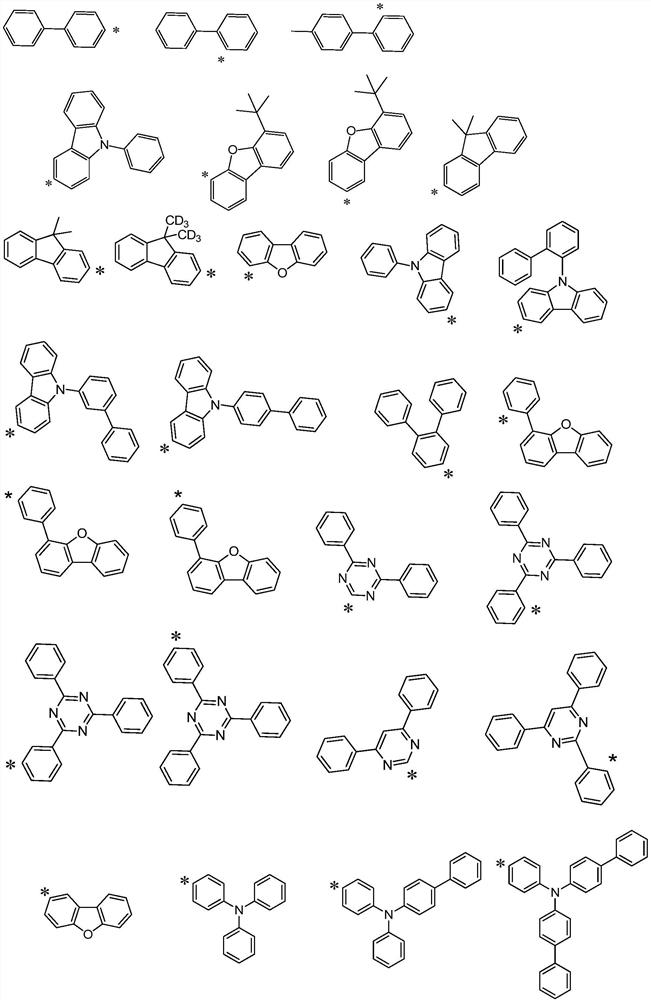
Hole transport material and organic electroluminescent device using same
A technology of hole transport materials and hole transport layers, which is applied in the fields of electric solid-state devices, electrical components, organic chemistry, etc., can solve the problem that the thermal stability of hole transport materials is lower than that of light-emitting layer materials or electron transport materials, and achieve Excellent carrier transport ability and stability, increased delocalization, and improved hole transfer rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040]
[0041] 1 is synthesized as follows:
[0042] (1)
[0043]
[0044] Compound 1 (405g / mol, 10g, 24.7mmol), FeCl 3 (0.2eq, 162.2g / mol, 4.94mmol, 0.8g), CS 2 (200g, 20 times the mass of compound 1) was added to the reaction flask, chlorobutane (2.1eq, 92.57g / mol, 51.87mmol, 4.8g) was added under ice cooling, and after the addition was completed, it was slowly returned to room temperature and reacted for 10h. , the reaction solution is poured into ice cubes (400g, the mass of ice cubes is CS 2 2 times the mass of ), hydrochloric acid was added dropwise until the pH of the system reached 2-3, and then dichloromethane (400g, CS 2 2 times the mass of the compound) for extraction, separated the dichloromethane phase and washed it several times with water, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain the crude product of compound 2, which was purified by column chromatography to obtain the pure product of compound 2 (8.77g, har...
Embodiment 2
[0055]
[0056] 2 is synthesized as follows:
[0057] Step 1-2 is basically the same as embodiment 1, and all the other steps are as follows:
[0058] (3)
[0059]
[0060] Under nitrogen protection, compound 4 (12g, 281g / mol, 42.7mmol), compound 7 (1eq, 321.15g / mol, 42.7mmol, 13.71g), sodium tert-butoxide (1.1eq, 96.1g / mol, 46.97mmol , 4.51g), Pd2(dba)3 (5%eq, 915.72g / mol, 2.14mmol, 1.96g), tri-tert-butylphosphine (5%eq, 202.317g / mol, 2.14mmol, 0.43g), toluene (120g, 10 times the mass of compound 4) was added to the reaction flask, and after the addition was completed, the temperature was raised to reflux for 12 hours. After the HPLC detection reaction was completed, after cooling down to room temperature, water was added and stirred for 15 minutes to obtain the filtrate, and the organic phase was obtained after the filtrate was separated. , the organic phase was dried with anhydrous magnesium sulfate and passed through a silica gel funnel to obtain a secondary filtra...
Embodiment 3
[0065]
[0066] 3 is synthesized as follows:
[0067] Step 1-2 is basically the same as embodiment 1, and all the other steps are as follows:
[0068] (3)
[0069]
[0070] Under nitrogen protection, compound 4 (12g, 281g / mol, 42.7mmol), compound 9 (1eq, 378.22g / mol, 42.7mmol, 16.15g), sodium tert-butoxide (1.1eq, 96.1g / mol, 46.97mmol , 4.51g), Pd2(dba)3 (5%eq, 915.72g / mol, 2.14mmol, 1.96g), tri-tert-butylphosphine (5%eq, 202.317g / mol, 2.14mmol, 0.43g), toluene (120g, 10 times the mass of compound 4) was added to the reaction flask, and after the addition was completed, the temperature was raised to reflux for 12 hours. After the HPLC detection reaction was completed, after cooling down to room temperature, water was added and stirred for 15 minutes to obtain the filtrate, and the organic phase was obtained after the filtrate was separated. , the organic phase was dried with anhydrous magnesium sulfate and passed through a silica gel funnel to obtain a secondary filtra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com