Application of VDAC protein to cell growth inhibition by utilizing oxalic acid or calcium salt and earthquake prediction
A technology of earthquake prediction and oxalic acid, which is applied in the direction of antibacterial drugs, resistance to vector-borne diseases, peptide sources, etc., can solve the problems of timely and accurate earthquake prediction, and achieve the effect of preventing and mitigating natural disasters
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
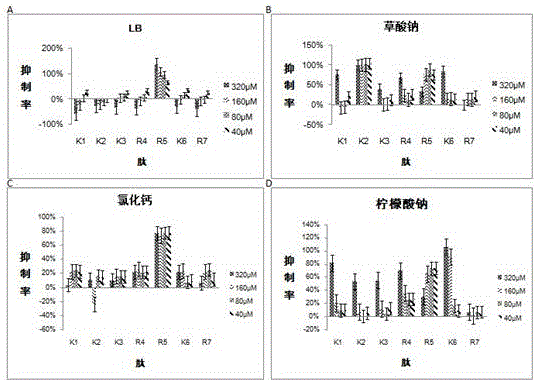
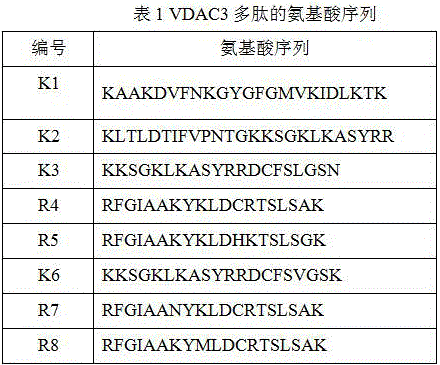
[0028] Example 1 Detection of the inhibition rate of 7 peptides to Escherichia coli MG1655
[0029] Each synthetic peptide was dissolved in sterile water so that the stock solution concentration of the peptide was 20 mM. Then, each peptide was diluted to four final concentrations with four corresponding liquid media to form peptide solutions, which were 40 μM, 80 μM, 160 μM, and 320 μM, respectively. Dilute Escherichia coli to 4*10 5 cfu / ml final concentration. According to Table 2, the bacterial solution and the peptide solution were spotted on a 96-well plate, and three parallel controls were set for each spotting well. According to the culture time of Escherichia coli determined before, put the 96-well plate with the sample in the incubator at 37°C for 20 hours and measure the OD value at 492nm with a microplate reader. According to the measured OD value, the antibacterial rate of various peptides against Escherichia coli in different concentrations and different media w...
Embodiment 2
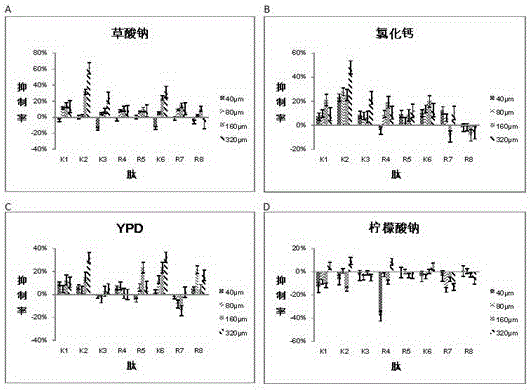
[0035] Example 2 Detection of the inhibition rate of 8 kinds of peptides to Saccharomyces cerevisiae INVSc1
[0036] Each synthetic peptide was dissolved in sterile water so that the stock solution concentration of the peptide was 20 mM. Then, each peptide was diluted to four final concentrations with four corresponding liquid media to form peptide solutions, which were 40 μM, 80 μM, 160 μM, and 320 μM, respectively. Dilute Saccharomyces cerevisiae to 1*10 3 cfu / ml final concentration. According to Table 3, the bacterial solution and the peptide solution were spotted on a 96-well plate, and three parallel controls were set for each spotting well. According to the previously determined yeast culture time, place the 96-well plate with the sample in a 30°C incubator for 21 hours and measure the OD value at 492nm with a microplate reader. According to the measured OD value, the bacteriostatic rate of various peptides against yeast in different concentrations and different media...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com