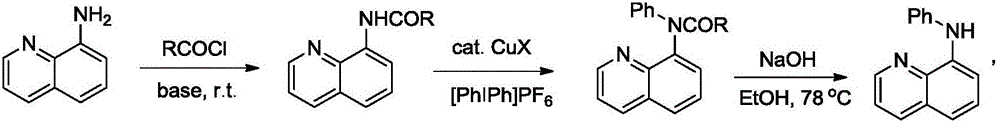Preparation method of N-phenyl-8-aminoquinoline
A technology of aminoquinoline and phenyl, which is applied in the field of preparation of N-phenyl-8-aminoquinoline, can solve problems such as limiting industrial application, and achieve the effect of simple operation and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment one: the preparation of a kind of N-phenyl-8-aminoquinoline derivatives, its synthetic route is:
[0024]
[0025] Embodiment 1: A kind of preparation method of N-phenyl-8-aminoquinoline derivatives is carried out according to the following steps:
[0026] (1) At room temperature, mix 8-aminoquinoline (1.0eq, 10.0mmol), benzoyl chloride (1.1eq, 11mmol), Et 3 N (1.2eq, 1.66mL), DMAP (0.3eq, 3mmol) were reacted in dichloromethane under nitrogen. After 12 hours, the reaction was quenched by adding water, extracted with dichloromethane, dried over magnesium sulfate, and concentrated by rotary evaporation. Column separation gave the target product (2.2300g, 90%).
[0027] (2) Take the target product I (1.0eq, 1mmol) of the previous step, copper acetate monohydrate (0.2eq, 0.2mmol), [PhIPh]PF 6 (2.0eq, 2mmol) into 1,2-dichloroethane, reacted in an oil bath at 80°C for 48h, and performed TLC detection. After the reaction was completed, add water to quench the r...
Embodiment 2
[0029] Embodiment two: the preparation of a kind of N-phenyl-8-aminoquinoline derivative, its synthetic route is:
[0030]
[0031] Embodiment two: the preparation of a kind of N-phenyl-8-aminoquinoline derivative, its synthetic route is:
[0032] (1) At room temperature, mix 8-aminoquinoline (1.0eq, 10.0mmol), o-methylbenzoyl chloride (1.1eq, 11mmol), Et 3 N (1.2eq, 1.66mL), DMAP (0.3eq, 0.366g) were reacted in dichloromethane under nitrogen. After 12 hours, the reaction was quenched by adding water, extracted with dichloromethane, dried over magnesium sulfate, and concentrated by rotary evaporation Column separation was performed to obtain the target product (2.2500 g, 86%).
[0033] (2) Take the target product I (1.0eq, 1.0mmol) of the previous step, copper acetate monohydrate (0.2eq, 0.2mmol), [PhIPh]PF 6 (2.0eq, 2.0mmol) into 1,2-dichloroethane, reacted in an oil bath at 80°C for 48h, and performed TLC detection. After the reaction was completed, add water to quench ...
Embodiment 3
[0035] Embodiment three: the preparation of a kind of N-phenyl-8-aminoquinoline derivative, its synthetic route is:
[0036]
[0037] Embodiment three: the preparation of a kind of N-phenyl-8-aminoquinoline derivative, its synthetic route is:
[0038] (1) At room temperature, mix 8-aminoquinoline (1.0eq, 10.0mmol), p-fluorobenzoyl chloride (1.1eq, 11mmol), Et 3 N (1.2eq, 1.66mL), DMAP (0.3eq, 3mmol) were reacted in dichloromethane under nitrogen. After 12 hours, the reaction was quenched by adding water, extracted with dichloromethane, dried over magnesium sulfate, and concentrated by rotary evaporation. Column separation gave the target product (2.3400g, 88%).
[0039] (2) Take the target product I (1.0eq, 1mmol) of the previous step, copper acetate monohydrate (0.2eq, 0.2mmol), [PhIPh]PF 6 (2.0eq, 2mmol) into 1,2-dichloroethane, reacted in an oil bath at 80°C for 48h, and performed TLC detection. After the reaction was completed, add water to quench the reaction, extract ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com