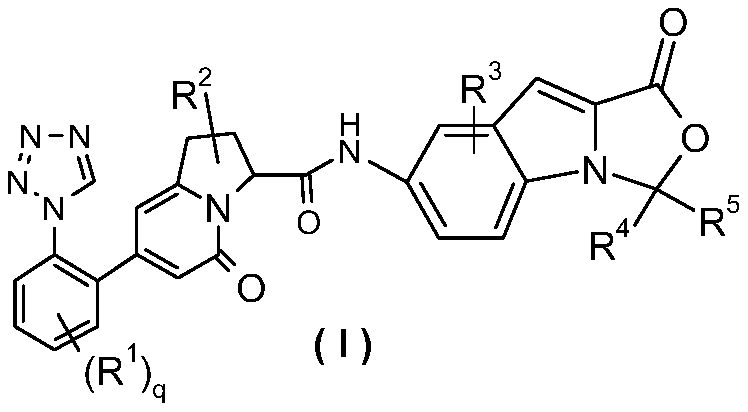
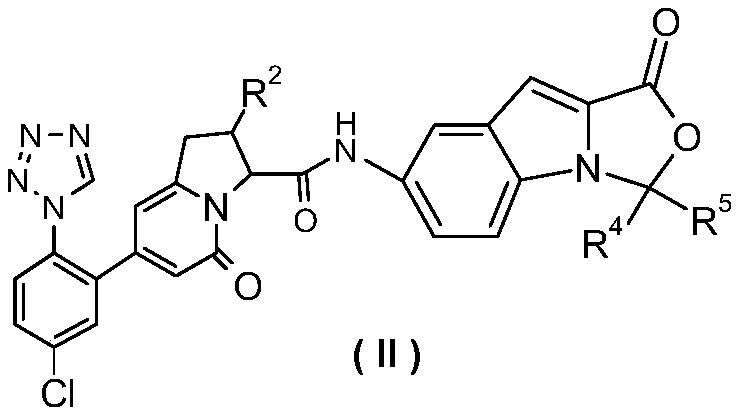
Oxazoloindole derivatives, their preparation method and their application in medicine
A technology of drugs and compounds, which is applied in the field of oxazoloindole derivatives, their preparation and their application in medicine, and can solve the problems of high cytotoxicity, excessive hydrophilicity or lipophilicity, adverse reactions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
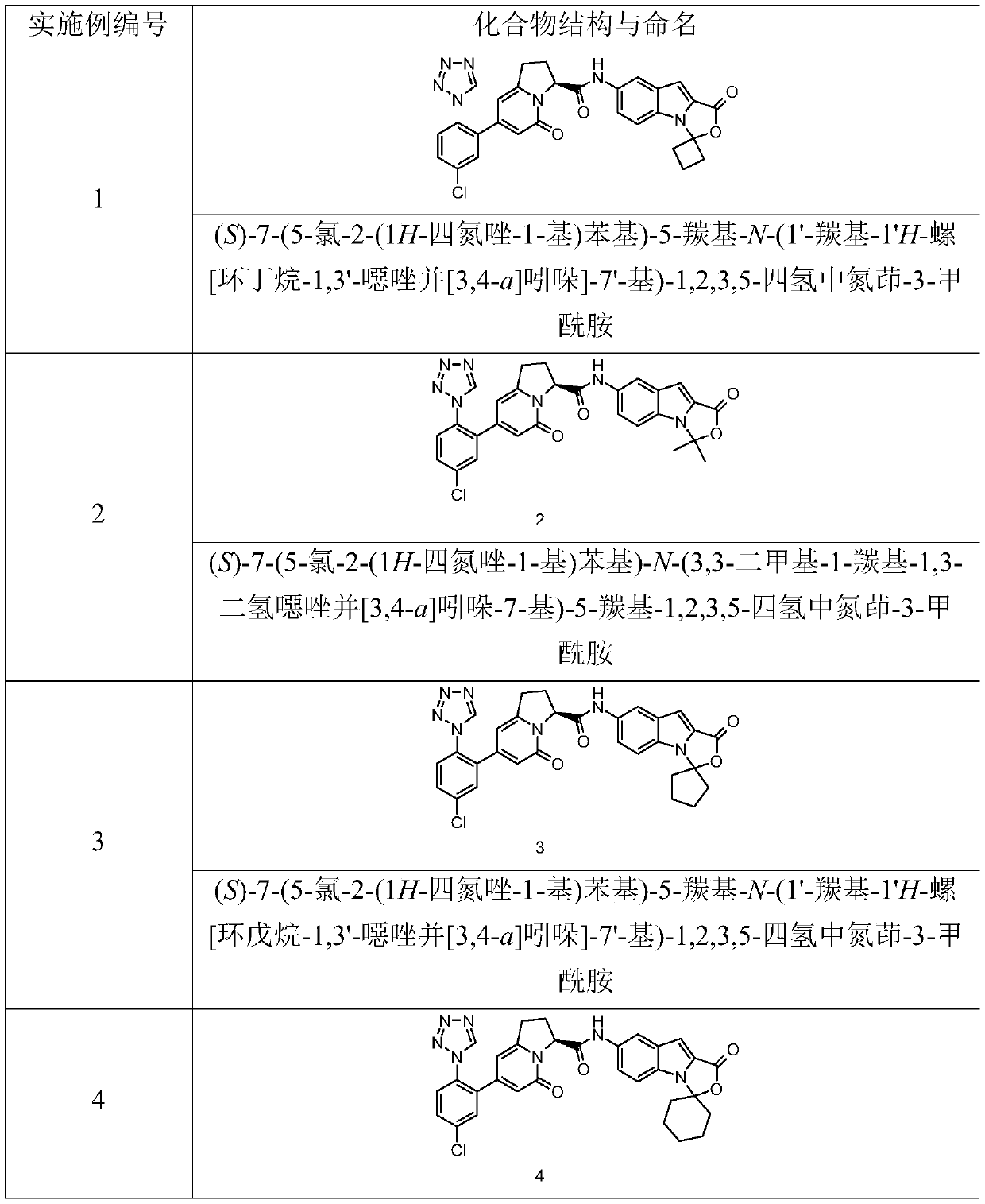
[0114] (S)-7-(5-chloro-2-(1H-tetrazol-1-yl)phenyl)-5-carbonyl-N-(1'-carbonyl-1'H-spiro[cyclobutane- 1,3'-oxazolo[3,4-a]indol]-7'-yl)-1,2,3,5-tetrahydroindolizine-3-carboxamide
[0115]
[0116] first step
[0117] (1'-Carbonyl-1'H-spiro[cyclobutane-1,3'-oxazolo[3,4-a]indol]-7'-yl)carbamate tert-butyl ester
[0118] 5-((tert-butoxycarbonyl)amino)-1H-indole-2-carboxylic acid 1a (1.10g, 4mmol, using the known method "Journal of the American Chemical Society, 2007, 129 (17), 5384- 5390" prepared) was dissolved in 30mL tetrahydrofuran, N,N'-carbonyldiimidazole (1269mg, 8mmol) was added under ice-cooling, the reaction solution was raised to room temperature, stirred for 1.5 hours, and cyclobutanone 1b (700mg, 10mmol) was added , 1,8-Diazabicycloundec-7-ene (1.58 g, 10.4 mmol), stirred for 3 hours. Add 50mL water to the reaction solution, extract with ethyl acetate (50mL×3), collect the organic layer, wash with 50mL water and 50mL saturated sodium chloride solution successively, ...
Embodiment 2
[0130] (S)-7-(5-chloro-2-(1H-tetrazol-1-yl)phenyl)-N-(3,3-dimethyl-1-carbonyl-1,3-dihydrooxane Azolo[3,4-a]indol-7-yl)-5-carbonyl-1,2,3,5-tetrahydroindolizine-3-carboxamide
[0131]
[0132] first step
[0133] (3,3-Dimethyl-1-carbonyl-1,3-dihydrooxazolo[3,4-a]indol-7-yl)carbamate tert-butyl ester
[0134] 5-((tert-butoxycarbonyl)amino)-1H-indole-2-carboxylic acid 1a (500mg, 1.81mmol) was dissolved in 20mL tetrahydrofuran, and N,N'-carbonyldiimidazole (734mg, 4.52mmol), the reaction solution was raised to room temperature, stirred for 1.5 hours, added 10mL of acetone, 1,8-diazabicycloundec-7-ene (720mg, 4.70mmol), stirred for 3 hours. The reaction solution was concentrated under reduced pressure, and the resulting residue was purified with a CombiFlash flash preparation apparatus with eluent system B to obtain the title product (3,3-dimethyl-1-carbonyl-1,3-dihydrooxazolo[3, 4-a] Indol-7-yl) tert-butyl carbamate 2a (375 mg, yellow solid), yield: 65.6%.
[0135] MS m / z(ES...
Embodiment 3
[0145] (S)-7-(5-chloro-2-(1H-tetrazol-1-yl)phenyl)-5-carbonyl-N-(1'-carbonyl-1'H-spiro[cyclopentane- 1,3'-oxazolo[3,4-a]indol]-7'-yl)-1,2,3,5-tetrahydroindolizine-3-carboxamide
[0146]
[0147] first step
[0148] (1'-Carbonyl-1'H-spiro[cyclopentane-1,3'-oxazolo[3,4-a]indol]-7'-yl)aminomethyl tert-butyl ester
[0149]5-((tert-butoxycarbonyl)amino)-1H-indole-2-carboxylic acid 1a (500 mg, 1.81 mmol) was dissolved in 10 mL of tetrahydrofuran, and N,N'-carbonyldiimidazole (587 mg, 3.62mmol), the reaction solution was raised to room temperature, stirred for 1 hour, added cyclopentanone 3a (396mg, 4.71mmol), 1,8-diazabicycloundec-7-ene (0.7mL, 4.71mmol), Stir for 3 hours. Add 30mL water to the reaction solution, extract with ethyl acetate (30mL×3), wash with 30mL water and 30mL saturated sodium chloride solution successively, dry the organic phase with anhydrous sodium sulfate, filter, concentrate the filtrate under reduced pressure, and use CombiFlash to flash The resulting...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com