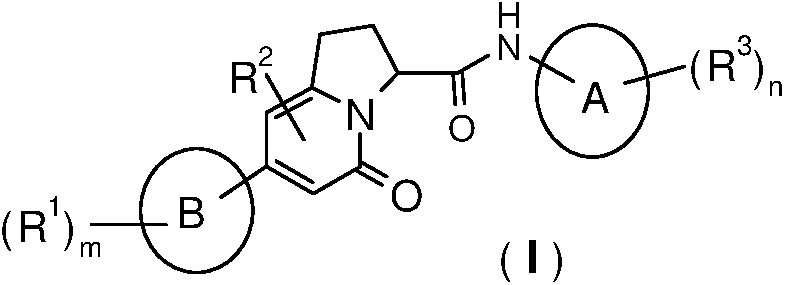
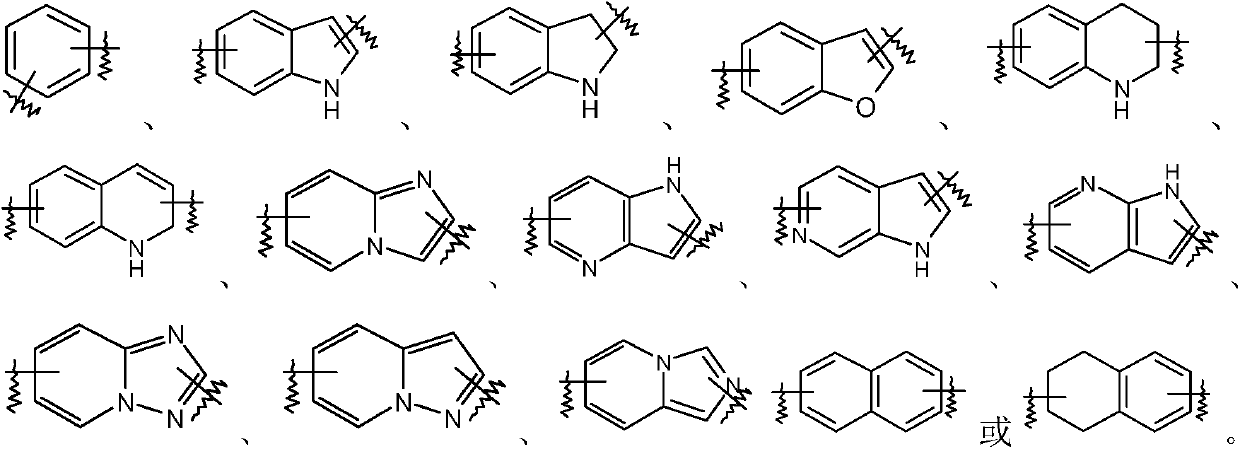
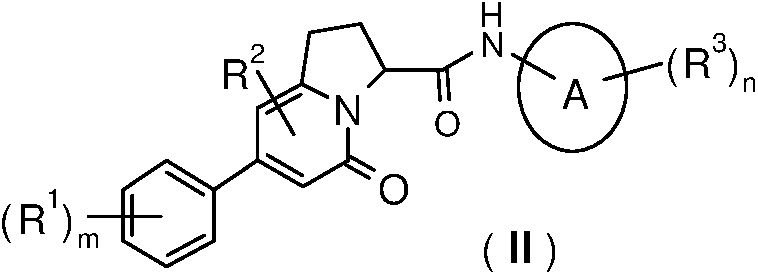
Indolizine-amide derivatives, their preparation method and their application in medicine
A compound and mixture technology, applied in the field of factor XIa inhibitors and disease drugs, can solve the problems of unpublished clinical results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0148] (S)-5-(7-(5-chloro-2-(1H-tetrazol-1-yl)phenyl)-5-oxo-1,2,3,5-tetrahydroindolizine- 3-amido)-1H-indole-2-carboxylic acid methyl ester
[0149]
[0150]
[0151] first step
[0152] (S)-5-(7-(2-Amino-5-chlorophenyl)-5-oxo-1,2,3,5-tetrahydroindolizine-3-amido)-1H-indole -2-Carboxylic acid methyl ester
[0153] 5-Amino-1H-indole-2-carboxylic acid methyl ester 1a (124 mg, 0.64 mmol, prepared by the known method "Journal of the American Chemical Society, 2007, 129 (35), 10858-10869"), (S)-7-(2-Amino-5-chlorophenyl)-5-oxo-1,2,3,5-tetrahydroindolizine-3-carboxylic acid 1b (200mg, 0.64mmol, using Prepared by the method disclosed in the patent application "WO2013093484"), 1-hydroxybenzotriazole (104mg, 0.77mmol), dicyclohexylcarbodiimide (160mg, 0.77mmol) were dissolved in 10mL N,N-dimethyl Formamide, stirred at room temperature for 16 hours. Filter and wash the filter cake with 20 mL of N,N-dimethylformamide. The mother liquors were combined and concentrated under re...
Embodiment 2
[0160] (S)-5-(7-(5-chloro-2-(1H-tetrazol-1-yl)phenyl)-5-oxo-1,2,3,5-tetrahydroindolizine- 3-amido)-1H-indole-2-carboxylic acid
[0161]
[0162] first step
[0163] (S)-5-(7-(5-chloro-2-(1H-tetrazol-1-yl)phenyl)-5-oxo-1,2,3,5-tetrahydroindolizine -3-amido)-1H-indole-2-carboxylic acid methyl ester 1 (23mg, 0.043mmol) was added to 2mL methanol and tetrahydrofuran (V / V=1:1) mixed solvent, and 0.2mL 2N hydroxide was added Lithium solution was stirred at room temperature for 2 hours. Concentrate under reduced pressure, add 10mL of water, add dropwise 2M hydrochloric acid to adjust the pH to 2~3, extract with dichloromethane and methanol (V / V=5:1, 30mL×3), combine the organic phases, wash with anhydrous sodium sulfate Dry, filter, and concentrate the filtrate under reduced pressure. Purification (HPLC) of the resulting residue by preparative separation gave the title product (S)-5-(7-(5-chloro-2-(1H-tetrazol-1-yl)phenyl)-5-oxo -1,2,3,5-tetrahydroindolizine-3-amido)-1H-indole...
Embodiment 3
[0167] (S)-7-(5-chloro-2-(1H-tetrazol-1-yl)phenyl)-N-(1H-indol-5-yl)-5-oxo-1,2, 3,5-Tetrahydroindolizine-3-carboxamide
[0168]
[0169]
[0170] first step
[0171] (S)-7-(5-chloro-2-(1H-tetrazol-1-yl)phenyl)-5-oxo-1,2,3,5-tetrahydroindolizine-3-carboxy acid
[0172] (S)-7-(2-amino-5-chlorophenyl)-5-oxo-1,2,3,5-tetrahydroindolizine-3-carboxylic acid 1b (60mg, 0.2mmol), Sodium azide (13 mg, 0.2 mmol) was dispersed in 0.3 mL of trimethyl orthoformate, and then 2 mL of glacial acetic acid was added dropwise, and the reaction was stirred at 65°C for 16 hours. Concentrate under reduced pressure, and purify the resulting residue by silica gel column chromatography with an eluent system to give the title product (S)-7-(5-chloro-2-(1H-tetrazol-1-yl)phenyl)-5 -Oxo-1,2,3,5-tetrahydroindolizine-3-carboxylic acid 3a (73 mg, gray solid), yield: 98.0%.
[0173] MS m / z(ESI): 358.1[M+1]
[0174] second step
[0175] (S)-7-(5-chloro-2-(5-chloro-2-(1H-tetrazol-1-yl)phenyl)-N-(1H-i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com