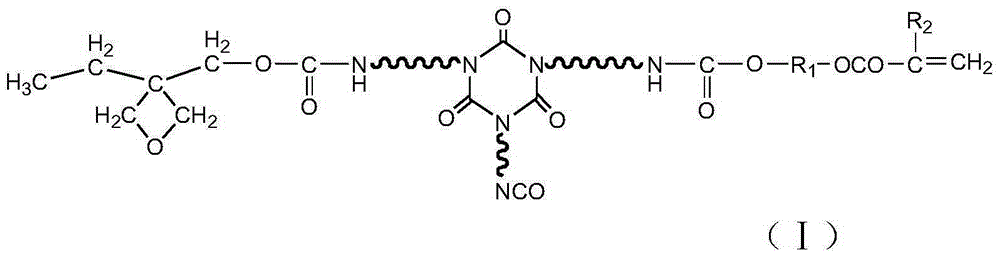
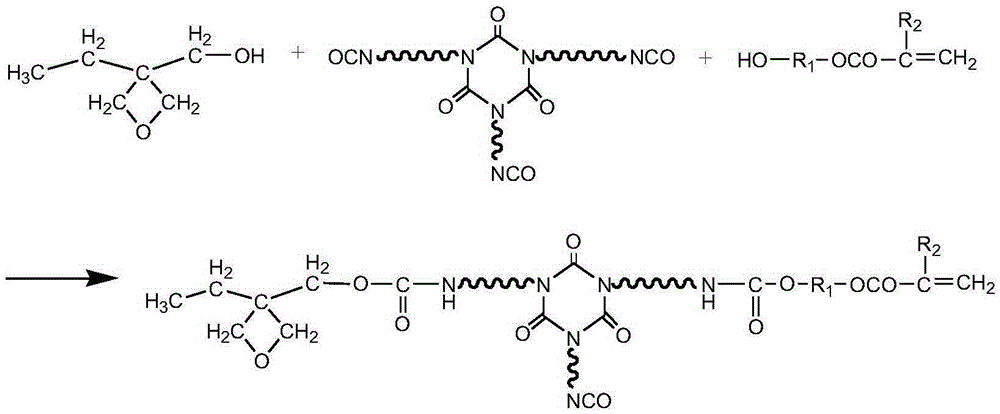
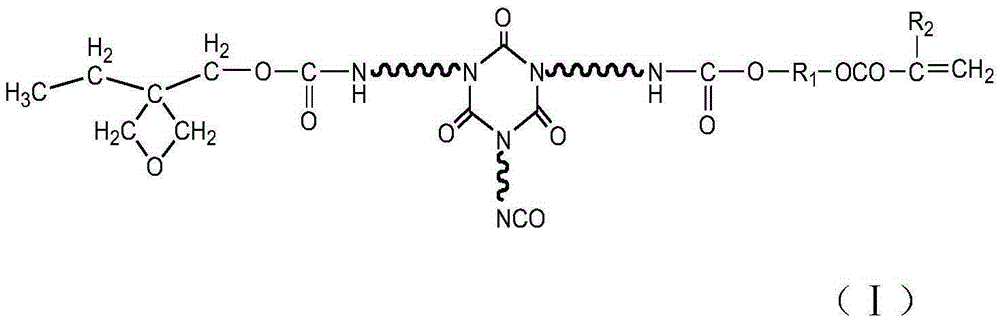
Multi-cured optical resin and synthesizing method thereof
A multi-curing, optical resin technology, used in organic chemistry, non-polymer organic compound adhesives, adhesives, etc., can solve the problems of LOCA not curing, incomplete curing, and unable to absorb UV light, and achieve good application value , The effect of low production cost and easy control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] HDI trimer (Bayer company, desmodur N3300) 80g, 3-ethyl-3-oxetanemethanol 10g, hydroxyethyl acrylate 4g, polymerization inhibitor p-hydroxyanisole 0.1g, catalyst dilauric acid di Add 0.01 g of butyltin into a three-necked flask with a thermometer, raise the temperature to 65 degrees, control the temperature at 75 degrees, and react for 2 hours. The NCO group is analyzed by an infrared spectrometer, and when the NCO peak does not change, the heating reaction is stopped to obtain a multi-curable optical resin.
Embodiment 2
[0029] HDI trimer (Bayer company, desmodur N3300) 110g, 3-ethyl-3-oxetanemethanol 5g, hydroxyethyl methacrylate 10g, polymerization inhibitor p-hydroxyanisole 0.1g, catalyst dilaurel Add 0.01 g of dibutyltin dibutyltin into a three-necked flask with a thermometer, raise the temperature to 65 degrees, control the temperature at 70 degrees, and react for 3 hours. The NCO group is analyzed by an infrared spectrometer, and when the NCO peak does not change, the heating reaction is stopped to obtain a multi-curable optical resin.
Embodiment 3
[0031] HDI trimer (Bayer, desmodur N3300) 100g, 3-ethyl-3-oxetanemethanol 8g, hydroxypropyl acrylate 6g, polymerization inhibitor p-hydroxyanisole 0.1g, catalyst dilauric acid di Add 0.01 g of butyltin into a three-necked flask with a thermometer, raise the temperature to 65 degrees, control the temperature at 73 degrees, and react for 2 hours. The NCO group is analyzed by an infrared spectrometer, and when the NCO peak does not change, the heating reaction is stopped to obtain a multi-curable optical resin.
PUM
| Property | Measurement | Unit |
|---|---|---|
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com