Polymorphic substances of triaryl dimethyl piperazine hydrobromide, and preparation method and application thereof
A technology of dimethylpiperazine and ketone hydrobromide, applied in the field of medicine, can solve problems such as inability to concentrate, sexual dysfunction, long onset time, and confusion of thinking, and achieve the prevention and treatment of depression and other potential diseases, good stability and high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1 (4-((R)-((2S,5R)-4-(3-fluorobenzyl)-(2,5-dimethylpiperazin-1-yl)(3-hydroxyphenyl) Preparation of polymorph A of methyl) phenyl) (4-methylpiperidin-1-yl) ketone hydrobromide
[0047] Take 2g of (4-((R)-((2S,5R)-4-(3-fluorobenzyl)-(2,5-dimethylpiperazin-1-yl)(3-hydroxyphenyl)methyl base) phenyl) (4-methylpiperidin-1-yl) ketone into a glass bottle, add 15 mL of acetone into the glass bottle, stir evenly, then add 1 mL of hydrobromic acid with a volume percentage of 40%, and stir at room temperature , reacted for 16 hours, filtered and dried to obtain (4-((R)-((2S,5R)-4-(3-fluorobenzyl)-(2,5-dimethylpiperazin-1-yl )(3-hydroxyphenyl)methyl)phenyl)(4-methylpiperidin-1-yl)methanone hydrobromide polymorph A.
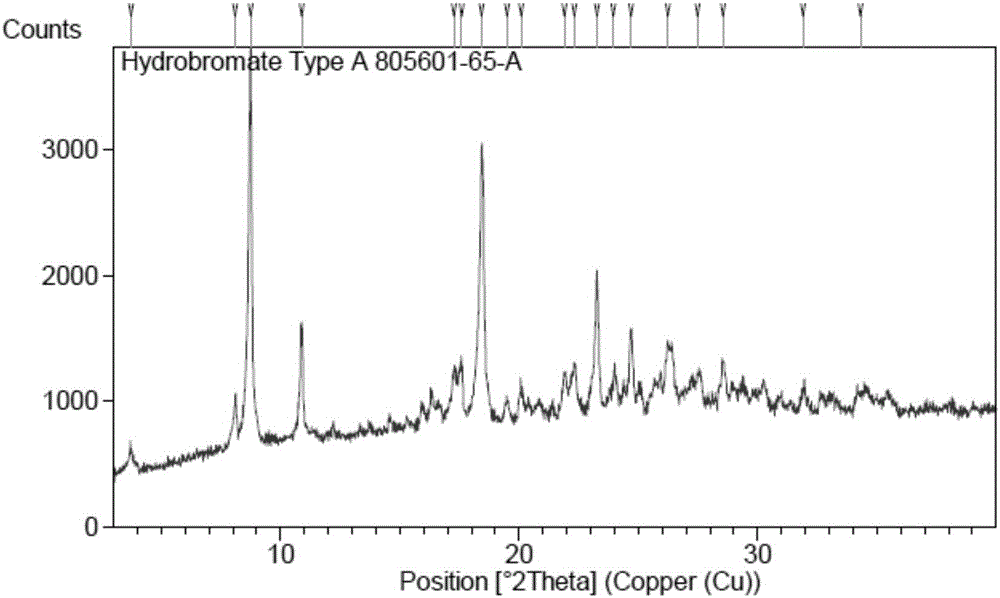
[0048] (4-((R)-((2S,5R)-4-(3-fluorobenzyl)-(2,5-dimethylpiperazin-1-yl)(3-hydroxy The XRPD pattern of polymorph A of phenyl) methyl) phenyl) (4-methylpiperidin-1-yl) ketone hydrobromide figure 1 The peak information of its spectrum is shown in Table 1. TGA ...
Embodiment 2
[0051] Example 2 (4-((R)-((2S,5R)-4-(3-fluorobenzyl)-(2,5-dimethylpiperazin-1-yl)(3-hydroxyphenyl) Preparation of polymorph B of methyl)phenyl)(4-methylpiperidin-1-yl)methanone hydrobromide
[0052] (4-((R)-((2S,5R)-4-(3-fluorobenzyl)-(2,5-dimethylpiperazin-1-yl)(3- The polymorph A of hydroxyphenyl)methyl)phenyl)(4-methylpiperidin-1-yl)methanone hydrobromide was used as the starting sample, heated to 100°C, and collected after constant temperature for 5 minutes The solid is polymorph B.
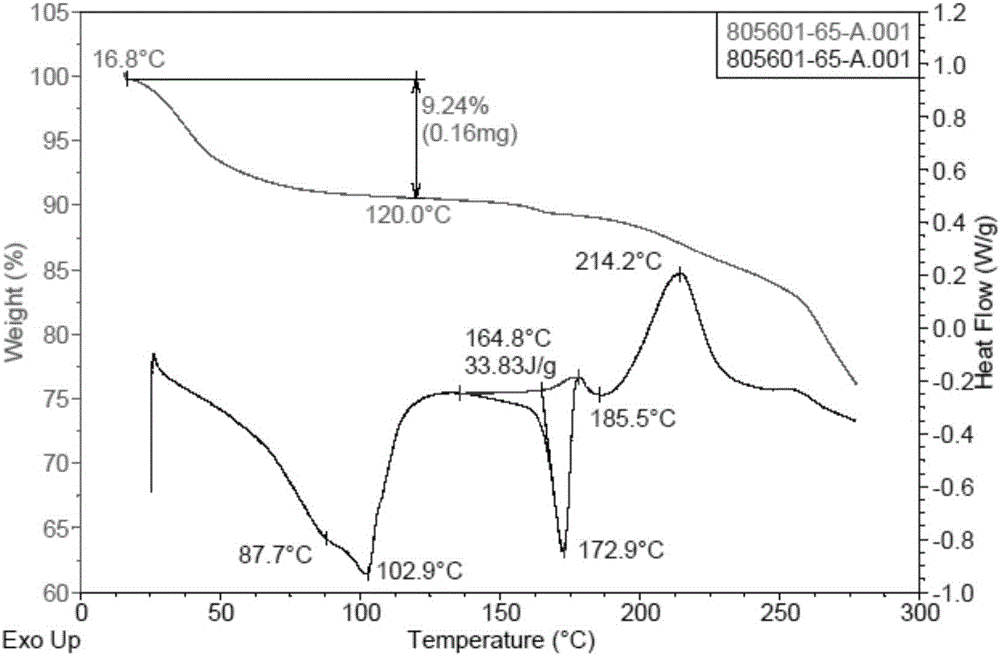
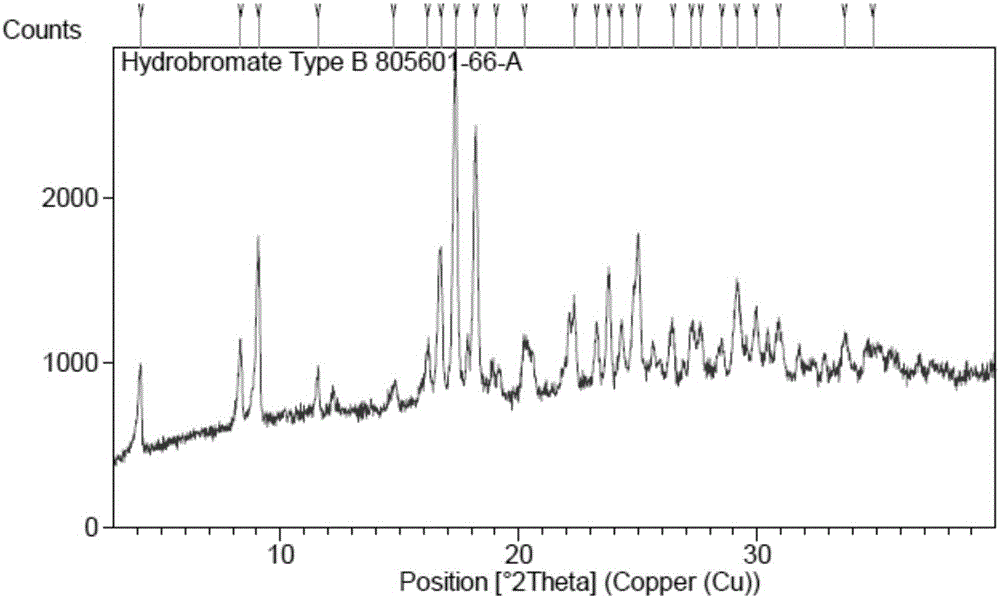
[0053] (4-((R)-((2S,5R)-4-(3-fluorobenzyl)-(2,5-dimethylpiperazin-1-yl)(3-hydroxy The XRPD pattern of polymorph B of phenyl)methyl)phenyl)(4-methylpiperidin-1-yl)methanone hydrobromide is shown in image 3 The peak information of its spectrum is shown in Table 2. TGA and DSC diagrams of polymorph B Figure 4 shown by Figure 4 It can be seen that the melting point of polymorph B is 174.2°C (the melting range is 165.8°C-174.2°C as judged from DSC).
[0054] Table 2 XRPD peak information...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com