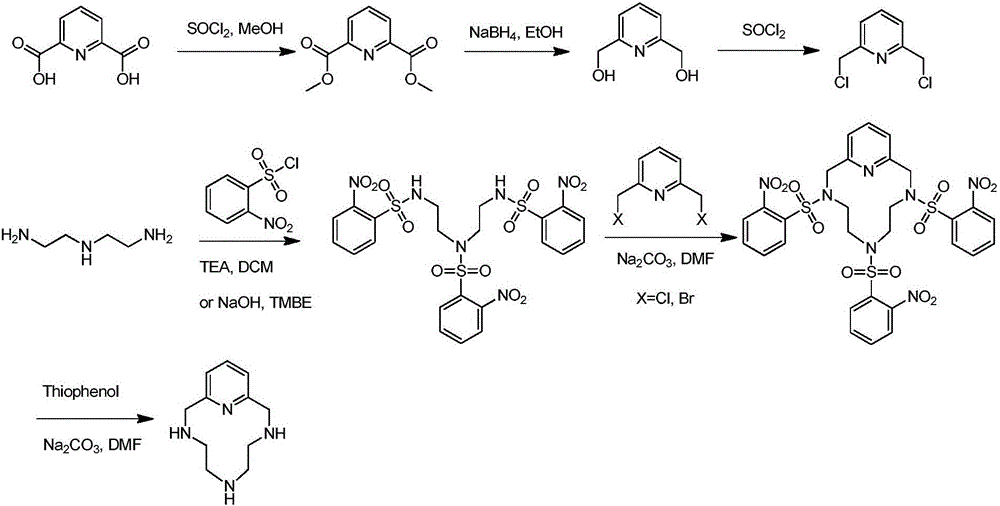
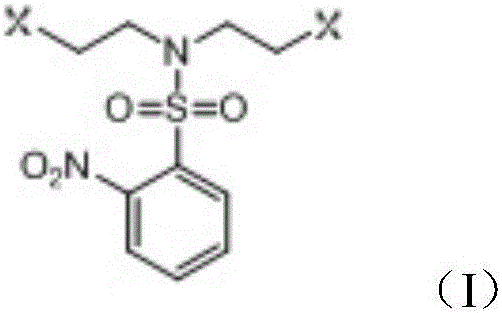
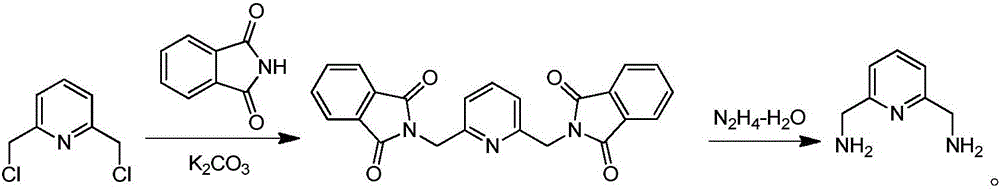
Method for preparing 1,4,7,10-tetraaza-2,6-pyridinophane
A technology of pyridine ring and tetraazine, which is applied in the field of medicine, can solve the problems of large steric hindrance, low yield, and difficulty in removing by-products, and achieve the effects of low price, high safety, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] S1: Add 115.7g (1.1mol, 1.1eq) of aminoethylene glycol and 80.0g (2.0mol, 2.0eq) of sodium hydroxide in 300L of water to a 2L four-neck flask with a mechanical stirrer and a thermometer and 1.2L of dichloromethane as a solvent, stirred for 1 hour, then added 221.6g of o-nitrobenzenesulfonyl chloride (1.0mol, 1.0eq) in three batches, slowly raised the temperature to between 25°C and 30°C, and continued to stir for 6h to complete reaction. Stand for stratification, separate the lower organic phase, wash the organic phase with 100mL 5% hydrochloric acid, concentrate the organic phase to about 500mL under reduced pressure, add 500mL n-heptane, a large amount of white solids are produced, filter with suction, and dry to obtain 274g of N,N-bis(2-hydroxyethyl)o-nitrobenzenesulfonamide, yield 94%, purity 99% (HPLC).
[0031] S2: Add 274g (0.944mol, 1.0eq) N,N-bis(2-hydroxyethyl)o-nitrobenzenesulfonate to a 2L dry four-necked flask equipped with a mechanical stirrer, a thermome...
Embodiment 2
[0038]S1: Add 126.2g (1.2mol, 1.2eq) of aminoethylene glycol, 60.0g (1.5mol, 1.5eq) of sodium hydroxide in 300L of water to a 2L four-neck flask with a mechanical stirrer and a thermometer and 1.2L of dichloromethane as a solvent, stirred for 1 hour, then added 221.6g of p-nitrobenzenesulfonyl chloride (1.0mol, 1.0eq) in three batches, slowly raised the temperature to between 25°C and 30°C, and continued to stir for 6h to complete reaction. Stand for stratification, separate the lower organic phase, wash the organic phase with 100mL 5% hydrochloric acid, concentrate the organic phase to about 500mL under reduced pressure, add 500mL n-heptane, a large amount of white solids are produced, filter with suction, and dry to obtain 280g of N,N-bis(2-hydroxyethyl)-p-nitrobenzenesulfonamide, yield 96%, purity 99% (HPLC).
[0039] S2: Add 435g (1.5mol, 1.0eq) N,N-bis(2-hydroxyethyl)p-nitrobenzenesulfonate to a 2L dry four-necked flask equipped with a mechanical stirrer, a thermometer, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com